Mini Review / Open Access
DOI: 10.31488/jjm.1000113
Adoptive immuno-cell therapy with antecedent surgery has superior actuarial survival to immuno-cell herapy without antecedent surgery for advanced cancers
GokiShindo*1, Takayoshi Endo1,Masamitsu Onda1,Yoju Miyamoto2, Toru Kaneko3, Shigenori Goto4
Meditopia Numazu Clinic,575-1 Okaisshiki, Numazu city, Shizuoka 410-0012, Japan
Shinyamanote Hospital, 3-6-1 Suwa-choHigashimatsuyama city, Tokyo 189-0021 Japan
Odaiba Nijihashi Clinic, Daiba clinic mall 2F,2-2-4 Daiba Minato-Ku, Tokyo 135-0091 Japan
Seta Clinic Tokyo, New Surugadai Bldg. 4F, 2-1-45 Kanda Surugadai, Chiyoda-Ku, Tokyo 101-0062, Japan
*Corresponding author:GokiShindo M.D., Meditopia Numazu Clinic, 575-1 Okaisshiki, Numazu city, Shizuoka 410-0012, Japan
Abstract
Adoptive immuno-cell therapy is considered one of the less toxic supportive therapies compared with conventional chemotherapy and radiotherapy for advanced cancers. Out of total 38 patients, 23 patients received surgery before immuno-cell therapy, 2 (8.7%) of 23 showed a complete response (CR) and 15 (65%) showed a partial response (PR) or prolonged stable disease (SD). Of the 15 remaining patientswith no antecedent surgery, there was no CR but 7 (46%) showed PR or prolonged SD.Actuarial survival analysis revealed that 23 patients with antecedent surgery afforded significantly longer survival than 15 patients without antecedent surgery (P<0.001). Antecedent surgical resection of tumors is recommended for obtaining better efficacy of immuno-cell therapy, even in advanced cancer patients.
Keywords: adoptive immuno-cell therapy, activated lymphocyte therapy, dendritic cell vaccination, therapy, immuno-chemotherapy, advanced cancer
Introduction
Improved immuno-cell therapyusing activated lymphocytes (ALs) with or without dendritic cell (DC) vaccination is performed at Mediotopia Numazu Clinic, Japan, as one of the supportive therapies for patients with advanced cancer and refractory cancer.Adoptive immuno-cell therapy has very low toxicity and exhibits fewer side effects than conventional chemotherapy and radiotherapy, as has been demonstrated over the past 20 years of clinical trials with immuno-cell therapy, including those conducted for “highly advanced medical technology’’ in some medical institutions under the approval of the Japanese Ministry of Health, Labor and Welfare.
Currently in Japan, immuno-cell therapy is an accepted and widely used treatment for advanced cancers to protect the patient’s immune system against tumor cells, but is at present not funded by the national health insurance system.
Patients and Methods
Patients and treatment
Patients with malignant cancer confirmed histologically at antecedent treatment underwent adoptive immuno-cell therapy at Meditopia Numazu Clinic between November 2007 and November 2010. Written informed consent was obtained from all patients before the start of immuno-cell therapy. The clinical data of38 consecutive patients (18 men, 20 women; mean age, 64 years; age range 39–83 years) were evaluated. The Eastern Cooperative Oncology Group (ECOG) performance status [1] at the beginning of the treatment was PS-0 in 18 patients, PS-1 in 17, PS-2 in 2, and PS-3 in 2. All patients underwent at least one course of treatment consisting of 6 infusions of ALs (3–10 x 109) and/or matured DCs (1–10 9×106) intravenously or sub-cutaneously at intervals of around 2 weeks. The patients also received additional conventional chemotherapy and/or radiotherapy in combination when indicated. The Seta Clinic Group, which supplies our clinic with ALs and DCs, has performed immuno-cell therapy [2]. The cell processing and immuno-cell therapy processing used were approved by the Ethics Committee of the Seta Clinic Group[3]. The generation and augmentation of ALs and the preparation of tumor lysates and peptides for DC vaccination were performed using lymphocytes and monocytes collected from peripheral blood. The precise processing of these cells is described previous papers [4–6].
Clinical responses and assessment
Clinical response was evaluated by physical and/or radiographic examination according to RECIST.A prolonged SD was defined as ‘‘long SD’’ that remained unchanged for more than 6 months.
Statistical analysis
Patients were evaluated for demographic, physical and laboratory variables. The distributions of these variables were compared by the chi-square test for qualitative vari- ables and Fisher’s Student t test for quantitative variables. Patient survival was analyzed using the Kaplan–Meier method. Differences in survival were determined using the log-rank test for univariate analysis. Statcel ver.2 for Windows Excel statistical software was used for all analyses.
Results
Patient characteristics
Among the 38 patients, the original site of advanced cancer was the lung in 5 (13.2%) patients, liver and bile duct in 4 (10.5%), pancreas in 8 (21%), esophagus in 4 (10.5%), stomach in 4 (10.5%), colon in 1 (2.6%), breast in 3(7.9%), uterus in 2 (5.2%), ovary in 4 (10.5%), and other sites in 3 (7.9%).
The disease was stage IV in 19 (50%) patients with distant metastases at various sites and stage III in 19 (50%) patients. Twenty-three (60.5%) of the total 38 patients had previously undergone curative surgery (antecedent surgery group), while the remaining 15 (39.5%) had not undergone surgery but had received chemotherapy and/or radiotherapy (non-antecedent surgery group). In the antecedent surgery group were 7 (36.8%) of the 19 patients with stage IV disease and 16 (84.2%) of the 19 patients with stage III disease. Staging grade was significantly different between the antecedent surgery group and non-antecedent surgery group (P<0.0034).
Clinical response
ALT alone or in combination with DCT
Of the total 38 patients, 8 (21.1%) were treated with immuno-cell therapy with ALT alone and 10 (26.3%) with ALT + DCT without additional conventional chemotherapy and/or radiotherapy, whereas the remaining 20 (52.6%) patients were treated with ALT in combination with the conventional therapies. All patients received 6 or more infusions of ALs with or without DC vaccinations subcutaneously. The tumor response to therapy was determined after one course of immuno-cell therapy, with a maintenance time of more than 6 months. The results of immuno-cell therapy with or without additional conventional chemotherapy and/or radiotherapy are shown in table 1.
Toxicity was assessed in all patients. Side effects due to immuno-cell therapy were rare, with no treatment-related toxicity apparent in any of the cycles of immuno-cell therapy.
Of the 8 patients treated with ALT alone, 4 (50%) patients achieved long SD, ranging from 7 to 27 months. Two of these patients died 24 months after completion of the therapy. These patients with long SD were all in the antecedent surgery group. There were no cases of CR or PR in the non-antecedent surgery group.
Of the 10 patients treated with ALT+DCT (Table 1), 1 (10%) achieved CR and 5 (50%) achieved long SD.
Nine (81.8%) of the 11 patients who underwent antecedent surgery achieved long SD, ranging from 7 to 32 months. On the other hand, among 5 (55.5%) of the 9 patients in the non-antecedent surgery group treated with ALT and conventional chemotherapy, only 1 (20%) patient with long SD survived 16 months.
Comparison of antecedent surgery and non-antecedent surgery group responses
The clinical characteristics of the antecedent surgery group and non-antecedent surgery group were compared statistically and are summarized in table 2. Patients in the antecedent and non-antecedent surgery groups had a variety of different advanced cancers. All patients had advanced cancers of stage III or stage IV. There was no significant difference in age distribution, but there was a significant difference between the two groups in terms of staging grade (P<0.0034).
The clinical benefits of immuno-cell therapy were estimated statistically by comparing the two groups using the chi-square test: the antecedent surgery group showed a significantly higher response rate (P<0.0092). This suggests important roles for the antecedent cytoreducing effect of surgery in minimizing tumor extension and preventing dissemination and metastasis of cancer cells to some extent even in patients with advanced cancer in various organs.
Median survival time of the antecedent surgery group and non-antecedent surgery group was 36 and 9 months,respectively. Moreover, analysis of the actuarial survival rate after immuno-cell therapy for the two groups revealed that the antecedent surgery group showed a significantly superior response, as determined by the log-rank test (P<0.001; Figure 1).
Table 1. Response to immuno-cell therapy with or without conventional chemotherapy and/or radiotherapy.
| ALT alone | ALT+DCT | ALT+ chemotherapy or radiotherapy | total | p value | |
|---|---|---|---|---|---|
| CR, PR +long SD, SD+PD | CR, PR+long SD, SD+PD | CR, PR+long SD, SD+PD | |||
| Antecedent surgery | 0, 4, 1 | 1, 3, 3 | 2, 9, 0 | 23 | p<0.01 |
| Non-antecedent surgery | 0, 0, 3 | 0, 2, 1 | 0, 5, 4 | 15 | |
| Total | 8 | 10 | 20 | 38 |
ALT, activated lymphocyte therapy; DCT, dendritic cell therapy; CR, complete response; PR, partial response; long SD, prolonged stable disease for more than 6 months; SD, stable disease;PD, progessive disease.
Table 2. Comparison of clinical data for antecedent surgery group and non-antecedent surgery group.
| Antecedent surgery group n=23 | Non-antecedent surgery group n=15 | P value | |
|---|---|---|---|
| Age (years) | 59.9 (39-81) | 66.7 (65-83) | p<0.0682 |
| Clinical stage, III vs IV | 17 vs 4 | 3 vs 12 | p<0.0034 |
| Time interval between recurrence and immuno-cell therapy (months) | 11.34 (1-62) | 5.2 (1-17) | p<0.0467 |
| No. of infusions (mean) | 8.13 (6-24) | 6.33 (6-13) | P<0.1580 |
| Efficacy rate: CR+PR+long SD vs SD+PD | 19 vs 4 | 7 vs 8 | p<0.0092 |
| Actuarial survival rate | log-rank test p<0.001 |
ALT, activated lymphocyte therapy; DCT, dendritic cell therapy; CR, complete response; PR, partial response; long SD, prolonged stable disease for >6 months; SD, stable disease; PD, progessive disease.
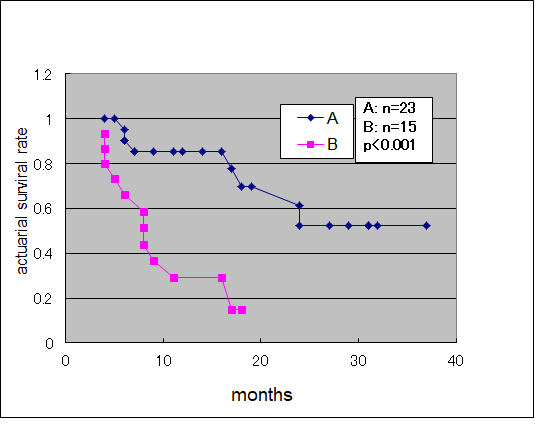
Figure 1.Actuarial Survival Curve of antecedent surgery group (A) and antecedent non-surgery group (B).
In 23 patients in the antecedent surgery group, there was a significantly (P<0.05) better response in the ALT andchemotherapy combination group (n = 7) than in the ALT+DCT group (n = 11). This finding suggests a certain benefit of immuno-cell therapy using ALs in combination with chemotherapy when planning the appropriate time interval between chemotherapy and immuno-cell therapy in order to prevent direct drug side effects on immuno-cells themselves.
Discussion
Many cytotoxic drugs that have been developed in the past 50 years are widely available for the treatment of advanced cancers. Although they are effective in some types of malignancies such as leukemia, malignant lymphoma, choriocarcinoma, and testicular carcinoma, most other solid and organ tumors such as those in the esophagus, stomach, lung, liver pancreas, and colon remain refractory to anticancer drugs. Cytotoxic drug therapies inevitably have toxic side effects. The goals of successful cancer therapy should be survival prolongation, symptom palliation, and improved quality of life for patients, especially when designing or evaluating immunotherapy trials because of delayed onset of effects. The high response rate reported for chemotherapy is the result of evaluation based on the temporary reduction in tumor size in accordance with RECIST criteria. However, this has not been shown to clearly correlate with duration of patient survival. Additionally, the tumor-reducing effect of the drug may be short-lived [10, 11]. Under many standard regimens, the median duration of the CR and PR responses is approximately 6 months and less than 12 months at most [12, 13].
Tumor reduction surgery for advanced-staged cancers has been tried widely for more than 10 years and positive results have been obtained with and without perioperative intraperitoneal chemotherapy, especially in the field of ovarian cancer, colorectal cancer, gastric cancer and other peritoneal cancers [14-16]. However, it has become clear through accumulated clinical experience that in order to improve the clinical benefits afforded by tumor reduction surgery with intraperitoneal chemotherapy, careful selection of patients is needed, and thus, the indication for this technique is strictly limited to a small number of patients with advanced cancer. On the contrary, adoptive immuno-cell therapy has almost no toxicity and can be used for patients with various types of cancer and can fundamentally be used with any cancer patients at any clinical stage without additional restrictive criteria for patient selection. Kamigaki T presented no serious adverse event in the prospective study of adoptive immune-cell therapy for patient with various type of advanced cancers [17]. Tumor reduction surgery with immuno-cell therapy would therefore be a treatment option that would extend the indications for surgery and is expected to increase the surgical benefits of tumor reduction surgery itself in various kinds of cancers compared with conventional tumor reduction surgery with chemotherapy.
In ALT and/or DCT immuno-cell therapy, no severe side effects were observed and favorable effects were noted in most patients; thus, even in patients with advanced cancer with a low performance status of PS-2 or PS-3 grade, adoptive immuno-cell therapy can be tolerated without any side effects. There were 2 (5.2%) patients with PS-2 and 2 (5.2%) with PS-3 who completed at least one course of the immuno-cell trial in this study.
Among the patients in the antecedent surgery group who received immuno-cell therapy, the ALT with chemotherapy subgroup showed a significantly higher response rate than the ALT + DCT subgroup. This may be due to the adjuvant quality of ALT overcoming the profound toxic effects of drug chemotherapy using appropriate time delays between ALT and anticancer drugs to prevent direct drug side effects on activated lymphocytes.
In the present study, antecedent curative surgery before immuno-cell therapy resulted in a favorable outcome, suggesting that antecedent curative surgery is an important factor for obtaining sufficient benefits from immuno-cell therapy through reducing the adverse actions of cancercells against the patient’s own cells, organs, and their activities. The superiority of immune-cell therapy with antecedent surgery to that with non-antecedent surgery in terms of actuarial survival rate confirmed the total chronological long-term benefits even for patients with advanced cancer.
Recentincreased number of clinical reports using adoptive immune-cell therapy with or without conventional chemotherapy and /or radiotherapy make it possible to analyze detail results according to each pathological type of cancer, e.g., lung cancer [18,19] ,gastric cancer[20,21], hepatic cancer[22] and glioma[23]. Almost all of reports show better efficacy of adoptive immune-cell therapy, with significant better overall survival rates and/or improvedprognosis of patients of each cancer.
In conclusion, although the number of patients treated in this preliminary study was small, statistical analysis clarified that surgery before immuno-cell therapy confers important benefits even for patients with advanced cancer. Such antecedent surgery is advisable in order to obtain higher clinical benefits for such patients in the future.
Moreover, detailed indications and criteria for each type of cancer should be examined and decided through large-scale studies.
Acknowledgements
We thank Dr J. Izeki and Dr M. Takagi of Shizuoka General Hospital, Dr K. Chihara of Shizuoka Municipal Hospital and Dr Y. Hirai of Fujieda Heisei Hospital for their valuable support.
References
Oken MM, Davis TE, Creech RH, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J ClinOncol. 1982;5:640–655
Egawa K. Immuno-cell therapy of cancer in Japan. Anticancer Res. 2004; 24:3321–3326
Egawa K. On the safety assurance of all processing carried out in medical institutions for autologous immuno-cell therapy. Hum Cell. 2004; 17:1–6
Goto S, Shirotani N, Hatakeyama M, et al. Clinical benefit of nontoxic therapy in patients with advanced cancer (Opinion). Anticancer Res. 2002; 22:2461–2464
Nieda M, Tomiyama M, EgawaK. Ex vivo enhancement of antigen-presenting function of dendritic cells and its application for DC-based immunotherapy. Hum Cell. 2003; 16:199–204
Goto S, Kaneko T, Miyamoto Y,et al.Combined immunocell therapy using activated lymphocytes and monocytes-derived dendritic cells for malignant melanoma. Anticancer Res. 2005; 25:3742–3746
Novellino PE, Castelli C, Parmiani G. A listing of human antigens recognized by T cells:March 2004 update. Cancer ImmunolImmunother. 2005; 54:187–207
Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. New Engl J Med. 2009; 361:947–957
Kaufman B, Mackey JR, Clemens MR, et al.Trastuzumab plus anastorozol versus9 anastrozol alone for treatment of postmenopausal women with human epidermal growth factor receptor2-positive, hormonereceptor-positive metastatic breast cancer:Results from the randomized phase III TAnDEM study. J ClinOncol. 2009; 27:5529–5537
Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004; 10:909–915
Kawakami Y, Fujita T, Matsuzaki Y, et al. Identification of human tumor antigens and its implications for diagnosis and treatment ofcancer. Cancer Sci. 2004; 95:784–791.
Postmus PE, Scagliotti G, Groen HJ, et al. Standard versus alternating non-cross-resistant chemotherapy in extensive small cell lung cancer: an EOGTC phase III trial. Eur J Cancer. 1996; 324:1498–1503.
Hirschowitz EA, Hiestand DM, Yannelli JR. Vaccines for lung cancer.J ThoracOncol.2006; 1:93–104.
Van der Burg MEL, van Lent M, Buyse M, et al. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. N Engl J Med. 1995; 332:629–634.
al-Shammaa HAH, Li Y, Yoneyama Y. Current status and future strategies of cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. World J Gastroenterol.2008; 14:1159–1166.
Yan TD, Sim J, Morris DL. Selection of patients with colorectal peritoneal carcinomatosis for cytoreductive surgery and perioperative intraperitoneal chemotherapy. Ann SurgOncol. 2007; 14:1807–1817.
Kamigaki T, Matsuda E, Okada S, et al. Prospective evaluation of safety of immune-cell therapy for patients with various types of advanced cancer.Anticancer Res. 2014; 34:4601-4608.
Iwai K, Soejima K, Kudoh S, et al. Extended survival observed in adoptive activated T lymphocyte immunotherapy for advanced lung cancer: Results of multicenter historical cohort study. Cancer ImmunoloImmunother. 2012; 61:1781-1790
Kimura H, Matsui Y, Ishikawa A, et al. Randomized controlled phase 3 trial of adjuvant chemo-immunothrapy with activated αβkiller T cells and dendritic cells in patients with resected primary lung cancer. Cancer ImmunolImmunother. 2015; 64:51-59
Shen D, Liu ZH, Xu JN, et al. Effective adoptive cellular therapy in patients with gastric cancer: A meta-analysis.Immunotherapy. 2016; 8:971-981
Takimoto R, Kamigaki T, Okada S, et al. Efficacy of adoptive immune- cell therapy in patients with advanced gastric cancer: A retrospective study. Anticancer Res. 2017; 37:3947-3954
Takayama T, Sekine T, Makuuchi S, et al. Adoptive immunotherapy to lower postsurgical reccurence rates of hepatocellular carcinoma: Randomized trial. Lancet. 2000; 356:(9232) 802-807
Kenemaru Y, Sumida M, Okita Y, et al. Systemic intravenous transfer of autologous lymphokine-activated αβT-cells improves temozolomide-induced lymphopenia in patients with glioma.Anticancer Res. 2017; 37(7):3921-3932.
Received: April 20, 2018;
Accepted: May 11, 2018;
Published: May 12, 2018
To cite this article : Shindo G, Endo T, Onda M, et al. Adoptive immuno-cell therapy with antecedent surgery has superior actuarial survival to immuno-cell herapy without antecedent surgery for advanced cancers. Japan Journal of Medicine. 2018: 1:3.
© Shindo G, et al. 2018.
