Research Article / Open Access
DOI: 10.31488/jjm.1000144
Comparative Investigations on the Inhibition of Mobile Phone Radiation by Multiple Compartment Cavity Resonance Devices
Peter C. Dartsch*1, Florian M. König2
Dartsch Scientific GmbH, Institut für zellbiologische Testsysteme, Auf der Vosshardt 25, D-49419 Wagenfeld, Germany
Florian König Enterprises (FKE) GmbH, Gärtnerweg 48, D-86825 Bad Wörishofen, Germany
*Corresponding author:Prof. Dr. Peter C. Dartsch, Dartsch Scientific GmbH, Institute for Cell Biological Test Systems, Auf der Vosshardt 25, D-49419 Wagenfeld, Germany, Tel: +49 5444 980 1322;
Abstract
Mobile phone modulation technologies like LTE, UMTS and GSM are now fully established. Among these, mobile phones have been considered to emit only a radiation with low intensities when they are actively working and are placed near to the head. However, the safety aspects are not cleared evaluated. To address this topic, we have already investigated the cellular effects of DECT base radiation and its compensation by multiple compartment cavity resonance devices (RD). Prompted by this background, this study compares the efficacy of a second-generation RD with an optimized design in comparison to the first-generation RD used in previous studies. Connective tissue fibroblasts (cell line L-929) were exposed to the radiation of an actively transmitting commercially available mobile phone with 1.25 W/m2 at the level of the cells with and without the two different RD directed crosswise towards the cell layer during mobile phone irradiation. Unexposed cells in a similar incubator served as corresponding controls. The resulting cell vitality was checked by measurement of the enzymatic activity of mitochondrial dehydrogenases by the color change of the sodium salt 2,3-bis[2-methoxy-4-nitro-5-sulfo-pheny]-2H-tetrazolium-5-carboxyanilide (XTT). The results clearly demonstrate that exposure to mobile phone radiation caused a significantly reduced cell vitality by more than 50 % for non-thermal radiation with an intensity of 1.25 W/m2 at the level of the cells. The decreased cell vitality after mobile phone radiation exposure could be largely compensated by use of two crossed RD directed towards the cells. However, the newly designed RD construction was even more effective (reduction of cell vitality by 13.01 ± 1.86 %; mean value ± standard deviation) than the standard RD (reduction of cell vitality by 20.17 ± 2.16 %; mean value ± standard deviation) which has been already used successfully to compensate digitally enhanced cordless telecommunication (DECT) phone base station radiation.
Keywords: electromagnetic radiation, mobile phone modulation (LTE, UMTS, GSM), health effects, cell culture
Introduction
Mobile phones due to their modulation transmission standards such as (LTE, UMTS, GSM), digitally enhanced cordless telecommunication (DECT) phones, routers and others belong to a group of wireless telecommunication sources which have caused a dramatic increase in environmental levels of electromagnetic radiation [1,2]. All these sources emit radiation with different characteristics in a wide spectrum of frequencies ranging from 0.9 to > 5 GHz. Although the energy of this type of radiation is quite weak, recent research studies have provided strong evidence that electromagnetic radiation influences human wellbeing and health by affecting biological and biochemical processes [3-9]. Due to its world-wide importance with more than 5 billion users [10], the technology has been extensively investigated for ist health effects at the cellular, experimental animal, and epidemiological level. Epidemiological and experimental research on radiation device exposure which might be also potentially harmful to millions of people has become very extended and includes more than 1,670 peer-reviewed papers about electromagnetic fields published in scientific journals from 1979 through 2018 [11]. Moreover, the use of 5G will substantially increase the exposure to radiofrequency electromagnetic fields on top of the 2G, 3G, 4G, Wi-Fi, etc. for telecommunications already in place [12].
The objective of the present study was to investigate whether the newly constructed resonance devices (nRD) might be even more effective in neutralizing mobile phone radiation than the standard resonance devices (sRD) already presented recently [13].
Materials and Methods
Cell culture and experimental design
In the present study, cultured connective tissue fibroblasts (cell line L-929; Leibniz-Institut, Deutsche Sammlung für Mikroorganismen und Zellkulturen, Braunschweig, Germany) as a standard cell line for toxicological studies were taken at passages 62 to 80 over a total experimental period of approximately 3 months. Cells were routinely cultivated in the moist atmosphere of an incubator at 37 °C and gassed with 5 % CO2 and 95 % air to yield a constant pH value of 7.4. Culture medium was RPMI 1640 with 10 % growth mixture and standard amounts of gentamycin. All cell culture reagents were from Capricorn Scientific, 35085 Ebsdorfergrund, Germany.
For the tests, cells were seeded from 80 to 90 % confluent mass cultures at a density of 20,000 cells/well into 24 wells in the middle part of a 96 well-plate. After 24 hours to ensure cell attachment and metabolization, culture medium was exchanged to Leibowitz L-15 medium containing 10 % growth mixture and standard amounts of gentamycin. This culture medium guarantees a pH value at 7.4 at normal atmospheric conditions. Plates were transferred to an external mini incubator and cultivated further at 37 ± 1 °C without CO2 gassing. A commercially available and actively transmitting 1800 MHz mobile phone at continuous operation mode was used to conduct the exposure conditions. Radiation intensities were measured at the level of the cells at the same conditions used for the assays with an Aaronia Spectran HF-4060 equipped with a calibrated area antenna of 1 cm2. An intensity of 1.8 W/m2 was measured for the actively transmitting mobile phone at the level of the cells without an corrugated cardboard and 1.25 W/m2 when the same corrugated cardboard as used for previous cell experiments [13,14] was placed between the mobile phone and the cells in order to avoid any thermal influence by microwave radiation. Thus, when the corrugated cardboard was used, a value of 37.5 to 38 °C at the cover lid of the multiwell plates was measured. So we concluded that our experimental design omitted local thermal effects. The temperature in the incubator was kept constant at 37 ± 1 °C. All tests were conducted with unexposed control cells at the same cultivation conditions, but approximately 5 meters distant from the active mobile phone. Cell vitality was checked by morphological observation of the cell cultures and by enzymatic activity. For the second method, cell culture medium was removed and replaced by fresh culture medium containing 10 % of 2,3-bis[2-methoxy-4-nitro-5-sulfo-pheny]-2H-tetrazolium-5-carboxyanilide (XTT; Xenometrix AG, Allschwil, Switzerland) and incubated for 120 minutes in the incubator at 37 °C. Yellowish XTT is cleaved to an orange formazan by a complex cellular mechanism which occurs in viable cells only, and is related to NAD(P)H production by glycolysis. Therefore, the amount of formazan dye formed directly correlates to the number of metabolically active cells in the culture [15,16].
After 120 minutes, the optical density was measured as a differential measurement ΔOD = 450 – 690 nm after a 4 second shaking interval using an ELISA reader (BioTek Slx808 with software Gen5 version 3.0; Bad Friedrichshall; Germany). Statistical analysis of all test assays was done using the two-tailed Wilcoxon-Mann-Whitney test.
In the present study, the sRD and the nRD were directed crosswise to the cell samples (distance between tube end and cell layer was 100 mm) during mobile phone radiation in two completely independent experiments within 2 months. The observed cell vitality values were tabulated for each RD series and, finally, the percentage differences between sRD and nRD were calculated.
Resonance devices
Basically, the first generation of RD (sRD) consisted of passive elements or compartments with a length of around 35 cm and a tube diameter of 5 cm without any electronic parts. The RD described previously [13] was filled with layers of material of iron, zinc, copper, magnetized metal parts, cardboard, carbon or carbon related granulate materials and varying quartz granulate minerals. The use of hollow conductor elements was assumed from the usual high frequency electromagnetic signal transmission [12,13]. The use of carbon and quartz (SiO2) is well known by microelectronics (microprocessors) or semi-conductors and solar cells.
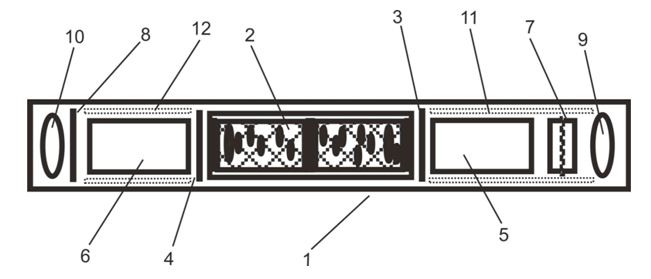
Figure 1.Schematic presentation of a standard resonance device consisting of a housing (1), a copper hollow conductor filled up with varying quartz granulates (2), carbon and zinced iron sheet (3), copper sheet (4), tube elements filled with quartz (5,6), magnet element (7), zinced iron sheet (8), rose quartz pieces (9,10) and some cardboard anti-shake elements (11,12). It consists of two additional AC power supply wires inside from right (input) to left (output) crossing the element number (2) as a copper/iron hollow conductor plus its wire surrounding elements (7,10). The novel resonance device has a water filled tube near to the rose quartz element (10) at the opposite side of the magnetized tube end.
The second generation and novel nRD construction also consisted of a tube filled with different layers or sheeting materials like sRD (cardboard, iron/zinc, copper magnetized metal parts, quartz, carbon as granulate). As depicted in Figure 1, the central element was a copper hollow conductor filled with quartz granulate mixtures. The conductor inlay was divided in two compartments by a central layer of carbon and different quartz granulates. Both types of tubes were closed up by a bigger rose quartz piece and a magnetized part at one tube end. Additionally, the nRD construction included the water filled tube near to the rose quartz element at the opposite side of the magnetized tube end.
Results and Discussion
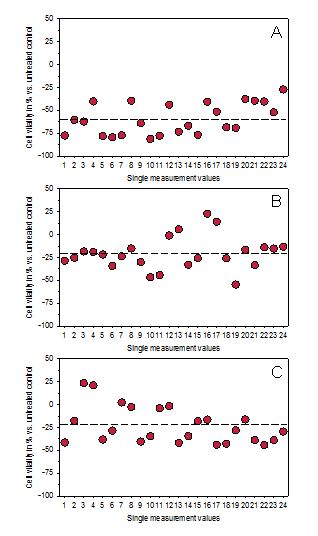
Figure 2.Graphical presentation of the original measurement data of cultured connective tissue fibroblasts exposed to non-thermal mobile phone radiation for 2 hours followed by a 22 h incubation period. (A) Cell vitality without any protective device and (B,C) cell vitality in two independent experiments by use of two standard resonance devices (sRD) directed crosswise towards the cells.
As already shown in a previous study for the exposure period of 2 h followed by a 22 h period of normal incubation, only 45.3 ± 5.3 % (mean value ± standard deviation; n = 13) of the cells survived for non-thermal radiation with an intensity of 1.25 W/m2 at the level of the cells [14]. One representative result of this series of experiments is depicted for comparative reasons in Figures 2A and 3A. This result clearly shows that non-thermal radiation is able to affect cell vitality even at relatively low intensities. This is in accordance to previous publications demonstrating that non-thermal effects also occur in biological systems and cause several alterations on the cellular level [17-21]. For example, Yakymenko et al. [21] have analyzed more than 100 available peer-reviewed scientific literature dealing with cellular effects induced by low-intensity radiofrequency radiation. They found a marked emphasis in the activation of key pathways generating reactive oxygen species, peroxidation and oxidative damage of DNA and changes in the activity of antioxidant enzymes suggesting a wide pathogenic potential of the induced reactive oxygen species and their involvement in cell signaling pathways. As also reviewed by Sage and Carpenter [22], exposure to electromagnetic fields has been linked to a variety of adverse health effects that might have significant public health consequences.
From this point of view, a device which is able to compensate at least part of the non-thermal radiation when using a mobile phone, might be very valuable. As depicted in Figures 2 B/C in form of the original measurements for each data point, the previous sRD was able to compensate non-thermal radiation of an actively transmitting mobile phone in the two test series by 20.64 and 23.70 %, respectively. Taken together, the sRD was able to inhibit reduction of cell vitality to 20.17 ± 2.16 % (mean value ± standard deviation). This value represents a significant compensation of non-thermal radiation by the actively transmitting mobile phone (p < 0.01; Wilcoxon-Mann-Whitney test).
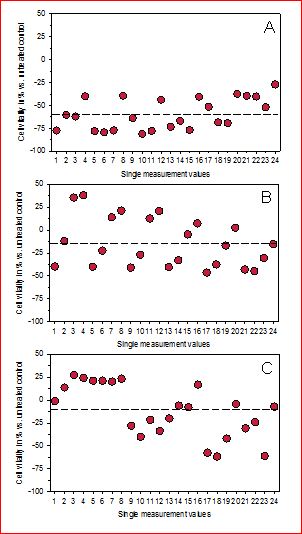
Figure 3.Graphical presentation of the original measurement data of cultured connective tissue fibroblasts exposed to non-thermal mobile phone radiation for 2 hours followed by a 22 h incubation period. (A) Cell vitality without any protective device and (B,C) cell vitality in two independent experiments by use of two newly designed resonance devices (nRD) directed crosswise towards the cells.
The novel nRD was even more effective (Figures 3 B/C) and was able to inhibit reduction of cell vitality to 14.31 % and 11.68 % in the two test series. This yields a summarized value for the inhibition of reduced cell vitality of 13.01 ± 1.86 %; mean value ± standard deviation). This value differs significantly from the value obtained for the sRD (p < 0.01; Wilcoxon-Mann-Whitney test). The difference is visualized for each data point in Figure 4 demonstrating that the nRD is even more effective than the previous sRD.
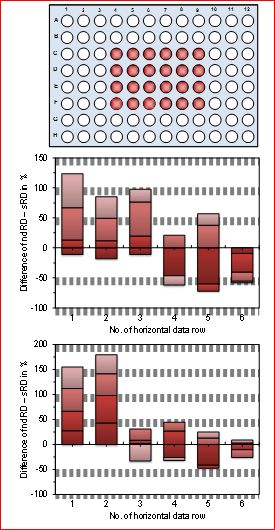
Figure 4.Graphical presentation as stacked sum of 4 columns and 6 rows as direct difference values of nRD minus sRD. The upper graphic represents the arrangement of the 4 columns and six rows in the 96-well plate which correspond to the difference values of nRD minus sRD in the two experimental series. A positive deviation represents a better compensation of nRD in comparison to sRD and a negative value the opposite. It can be easily seen that the nRD ist more efficient in compensating the mobile phone radiation. sRD = standard resonance device; nRD = newly designed resonance device.
Abbreviations: RD: resonance device(s); sRD: standard resonance device(s); nRD: newly constructed resonance device(s)
References
Ahlbom A, Feychting M. Electromagnetic radiation: Environmental pollution and health. Br Med Bull. 2003; 68: 157-165.
Sage C, Carpenter DO. Public health implications of wireless technologies. Pathophysiol. 2009; 16:233-246.
Diem E, Schwarz C, Adlkofer F, et al. Non-thermal DNA breakage by mobile-phone radiation (1800 MHz) in human fibroblasts and in transformed GFSH-R17 rat granulosa cells in vitro. Mut Res/Gen Toxicol Environ Mutagen. 2005; 583: 178-183.
Blank M, Goodman R. Electromagnetic fields stress living cells. Pathophysiol. 2009; 16: 71-78.
Hardell L, Carlberg M. Mobile phones, cordless phones and the risk for brain tumours. Int J Oncol. 2009; 35: 5-17.
Kundi M, Hutter HP. Mobile phone base stations – Effects on wellbeing and health. Pathophysiol. 2009; 16: 123-135.
Levitt BB, Lai H. Biological effects from exposure to electromagnetic radiation emitted by cell tower base stations and other antenna arrays. Environ Rev. 2013; 18: 369-395.
Vijayalaxmi, Scarfi MR. Biological health effects of radiofrequency fields. Int J Environ Res Public Health. 2014; 11: 9376-9408.
Hardell L, Carlberg M. 2017; Mobile phones, cordless phones and rates of brain tumors in different age groups in the Swedish National Inpatient Register and the Swedish Cancer Register during 1998-2015. PLoS ONE. 12(10): e0185461.
Davis DL, Kesari S, Soskolne CL, et al. Swedish review strengthens grounds for concluding that radiation from cellular and cordless phones is a probable human carcinogen. Pathophysiol. 2013; 20: 123-129.
Moskowitz JM. Electromagnetic Radiation Safety. Scientific and policy developments regarding the health effects of electromagnetic radiation exposure from cell phones, cell towers, Wi-Fi, smart meters, and other wireless technology. 2019. https://www.saferemr.com/2018/10/powerwatchlist.html. Visited on May 12, 2019.
Nyberg R, Hardell L. The 5G appeal. 2019. http://www.5gappeal.eu.
Dartsch PC, König FM. Neutralization of wireless DECT base radiation by novel resonance devices. Integr Mol Med. 2017; 4: 1-5.
Dartsch PC, Dochow T. Cellular effects following exposure to mobile phone radiation and its compensation. Jpn J Med. 2019; 2: 338-343.
Roehm NW, Rodgers GH, Hatfield SM, et al. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J lmmunol Meth. 1991; 142: 257-265.
Brosin A, Wolf V, Mattheus A, Heise H. Use of XTT-assay to assess the cytotoxicity of different surfactants and metal salts in human keratinocytes (HaCaT). A feasible method for in vitro testing of skin irritants. Acta Dermato-venereologica. 1997; 77: 26-28.
Funk RHW, Monsees TK. Effects of electromagnetic fields on cells: Physiological and therapeutical approaches and molecular mechanisms of interaction. Cells Tissues Organs. 2006; 182:59–78.
Funk RHW, Monsees T, Özkucur N. Electromagnetic effects – from cell biology to medicine. Progr Histochem Cytochem. 2009; 43:177-264.
Giuliani L, Soffritti M. Non-thermal effects and mechanisms of interaction between electromagnetic fields and living matter. Eur J Oncol, Library. 2010; 5.
Yakymenko I, Sidorik E, Henshel D, et al. Low intensity radiofrequency radiation: A new oxidant for living cells. Oxid Antioxid Med Sci. 2014; 3:1-3.
Yakymenko I, Tsybulin O, Sidorik E, et al. Oxidative mechanisms of biological activity of low-intensity radiofrequency radiation. Electromagn Biol Med. 2016; 35:186-202.
Sage C, Carpenter DO. Public health implications of wireless technologies. Pathophysiol. 2009; 16:233–246.
Received: April 22, 2019;
Accepted: May 23, 2019;
Published: May 27, 2019.
To cite this article : Dartsch PC, König FM. Comparative Investigations on the Inhibition of Mobile Phone Radiation by Multiple Compartment Cavity Resonance Devices. Japan Journal of Medicine. 2019: 2:3.
© Dartsch PC, et al. 2019.
