Mini Review / Open Access
DOI: 10.31488/jjm.1000133
Effects of dapagliflozin in type 2 diabetes patients with fatty liver
Koichi Takaguchi*1,Akemi Tsutsui1, Tomonori Senoh1, Ritsuko Yoshikawa1, Keigo Nakamura2, Atsushi Yoshida2, Takuya Nagano1
Department of Hepatology, Kagawa Prefectural Central Hospital, Takamatsu, Japan.
Department of Diabetes and Endocrinology, Kagawa prefetural Central Hospital
*Corresponding author:Koichi Takaguchi, Department of Hepatology, Kagawa Prefectural Central Hospital, 1-2-1 Asahi-machi,Takamatsu-city,Kagawa 760-8557, Japan, Tel: +81-87-811-3333; Fax: +81-87-802-1188;
Abstract
Aim: This study aimed to investigate the influences of a novel class of oral antidiabetic agent, sodium-glucose cotransporter 2(SGLT2) inhibitor,ontype 2 diabetes mellitus (T2DM)associated with fatty liver in the clinical setting.Methods: This was a retrospective, single-center, open-label, observational study conducted among Japanese outpatients with T2DM, who were newly treated with the SGLT2 inhibitor dapagliflozin in ourhospital. Fatty liver was determined using an abdominal ultrasonography or computed tomography. Changes in HbA1c, blood glucose, BMI, liver function parameters, liver stiffness, and steatosis evaluated by transient elastography during the 12-month study period were assessed.Results: A total of 117 patients with T2DM were enrolled in the study: 33 of them concomitantly had fatty liver. Male patients accounted for 57.6%, and mean age was 56.8 years in the patients with fatty liver. The mean HbA1c was 7.61% at baseline and significantly (p<0.01) reduced by -0.50% (-6.6% reduction) 12 months after treatment. The BMI and liver function parameters, such as aspartate aminotransferase (AST) and alanine aminotransferase (ALT), were also significantly reduced after the treatment (p<0.01).Transient elastography using FibroScan®, which measures liver stiffness or steatosis, indicated thatthere was no obvious change after treatment: however, changes inthese parameters were observed in one patient and were associated with changes in HbA1c and liver function parameters in this patient.Conclusion: In clinical setting, dapagliflozin improved HbA1c, reducing BMI and improving liver function parameters such as AST and ALT in Japanese T2DM patients with fatty liver.
Keywords: type 2 diabetes mellitus, fatty liver, nonalcoholic fatty liver disease, sodium-glucose cotransporter 2 inhibitor, dapagliflozin
Introduction
Globally, the number of patients with diabetes mellitus (DM) has dramatically increased in the past three decades, and DM is estimated to be the ninth major cause of death[1]. Type 2 DM (T2DM) accounts for approximately90% of adult DM worldwide[2,3]. Most patients with T2DM have at least one complication, and cardiovascular complications are the leading cause of morbidity and mortality[1]. Therefore, a broad range of interventionsfor not only the primary disease of T2DM but also for its complicationsismore strongly needed to prevent morbidity and mortality in patients with T2DM.
Nonalcoholic fatty liver disease (NAFLD) is a clinical syndromecharacterized by the accumulation of excess fat in the liver and the most common chronic liver condition of adults in developed countries[4,5].DM and NAFLD were clearly associated with each other, and they may serve as a progression factor for the other; recently, NAFLD is generally perceived as a benign condition, which may have on the contrary an important deleterious impact for diabetic patients increasing the risk of cardiovascular complications and serious hepatic diseases[6,7]. It has become critical for the health care providers to manage T2DM patients with NAFLD appropriately in clinical settings. However, no pharmacological treatments for this condition have been approved by regulatory agencies[8].
Recently, some new therapeutic options, such as incretin-based treatments and sodium-glucose cotransporter 2 (SGLT2) inhibitors, have been developed and clinically used for patients with T2DM[9]. SGLT2 inhibitorsarea novel class of oral antidiabetic agents that exert insulin-independent hypoglycemic effects by increasing urinary glucose excretion[10]. Effects of SGLT2 inhibitors on glucose control are well established, presenting a low risk of hypoglycemia, resulting in weight loss and lowering blood pressure[11-14]. It is also reported that SGLT2 inhibitors have various favorable effects on cardiovascular risks [15,16]. Additionally, several SGLT2 inhibitors have shown benefit in animal models of NAFLD[17,18], and some clinical studies have demonstrated improved liver enzyme activity, weight loss and reduced fatty liver index score in T2DM patients treated with SGLT2 inhibitors[19-23]. Therefore, SGLT2 inhibitors are expected to possibly become a useful pharmacological option for T2DM patients with NAFLD.
We investigated the influences of the SGLT2 inhibitor, dapagliflozin, on blood glucose, BMI, liver function parameters, liver stiffness and steatosis evaluated using transient elastography, and laboratory test parameters among Japanese T2DM patients with fatty liver in clinical setting.
Methods
Study design and patients
This was a retrospective, single-center, open-label, observational study on patients with T2DM, newly treated with dapagliflozin in clinical settings (UMIN000026549).
Outpatients with T2DM aged 20-84 years,who were began to treat with dapagliflozin between June 2014 and October 2016 at the Kagawa Prefectural Central Hospital in Japan, were recruited in this study. T2DM was diagnosed using a standard method according to the practice guideline for the treatment for diabetes in Japan 2016 published by the Japan Diabetes Society[24]. Dapagliflozin (5 mg) was administered once daily for 12 months as monotherapy or add-on therapy to other hypoglycemic agents (dipeptidyl peptidase-4 [DPP-4] inhibitor, sulfonylurea, biguanide, insulin, glucagon-like peptide-1 [GLP-1] receptor agonist, thiazolidine, glinide,or α-glucosidase inhibitor[α-GI]). Dapagliflozin was prescribed to patients whose blood glucose was inadequately controlled despite therapy with diet/exercise and other hypoglycemic agents (in the case of add-on therapy). The patients who had an allergy for dapagliflozin were excluded from the study.
Study assessments
Patients with T2DM were classified into two patient groups based on the presence or absence of fatty liver, determined using abdominal ultrasonography or computed tomography. In both patient groups (the total patient cohort and patients with fatty liver), changes in HbA1c, blood glucose, BMI, liver function parameters, liver stiffness and steatosis evaluated using transient elastography, and laboratory test parameters during the 12-month study period were assessed in the study. The liver function parameters evaluated included aspartate aminotransferase (AST), alanine aminotransferase (ALT) and γ-glutamyl transpeptidase (γ-GTP). The laboratory test parameters evaluated included total cholesterol, high density lipoprotein-cholesterol (HDL-C), low density lipoprotein-cholesterol (LDL-C), triglycerides, uric acid, blood urea nitrogen (BUN), creatinine, hematocrit and hemoglobin. These parameters were measured using conventional methods. Liver stiffness and steatosis were evaluated using a transient elastography,FibroScan® (Intermedical Co., Ltd., Tokyo, Japan), and the measurement data were expressed in E value (kilopascal: kPa) for liver stiffness index [25], and controlled attenuation parameter (CAP) value (dB/m) for liver steatosis index[26].
Ethics
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from the study patients, and the study protocol was approved by institutional review board of Kagawa Prefectural Central Hospital before the study initiation.
Statistical Analyses
Data were expressed as mean ± standard deviation (SD), number and percentage of patients or each individual measurement value. Statistical analyses were performed using BellCurve for Excel ver. 2.12 (Social Survey Research Information Co., Ltd., Tokyo, Japan).
Statistical comparison between baseline values and the valuesat 1, 3, 6, or 12 months after treatment were performed using the analysis of variance (ANOVA) model, adjusting the multiplicity with the Holm method. Missingdata were imputedusing the last observation carried forward. The paired t-testwas used for the statistical analysis of thechanges in laboratory test parameters after treatment. Differences were considered statistically significant at p values <0.05.
Results
Demographic and clinical characteristics of the patients
A total of 117 patients with T2DM were enrolled in the study. Among them, 33 patients concomitantly had fatty liver,as determined using abdominal ultrasonography or computed tomography. Patient characteristicsin these twogroups are summarized in Table 1. Male patients accounted for 58.1% and 57.6%, with a mean age of60.9 ± 12.9and 56.8 ± 14.3 years (data expressed as mean ± SD), and HbA1c of 7.63 ± 1.38% and 7.75 ± 1.58% in the total patient cohort and patients with fatty liver, respectively. Both patient groups also concomitantly had obesity, with a BMI of 29.3 ± 5.5 and 29.2 ± 5.1 kg/m2, respectively. Compared with the totalpatient cohort, the liver function parameters such as AST (40.4 ± 39.8 vs. 59.0 ± 41.2 IU/L), ALT (47.0 ± 55.4 vs. 80.8 ± 81.4 IU/L) and γ-GTP (63.9±102.3 vs.75.2±40.3 IU/L) were higher in patients with fatty liver.
Table 1. Patient characteristics.
| Total (N=117) | With fatty liver (N=33) | |||
|---|---|---|---|---|
| n | n | |||
| Male / female (n) [%] | 117 | 68 [58.1] / 49 [41.9] | 33 | 19 [57.6] / 14 [42.4] |
| Age (years) | 117 | 60.9±12.9 | 33 | 56.8±14.3 |
| BMI (kg/m2) | 59 | 29.3±5.5 | 30 | 29.2±5.1 |
| HbA1c (%) | 114 | 7.63±1.38 | 33 | 7.75±1.58 |
| Blood glucose (mg/dL) | 101 | 176.2±69.0 | 33 | 171.1±64.7 |
| AST (IU/L) | 115 | 40.4±39.8 | 33 | 59.0±41.2 |
| ALT (IU/L) | 115 | 47.0±55.4 | 33 | 80.8±81.4 |
| γ-GTP (IU/L) | 112 | 63.9±102.3 | 33 | 75.2±40.3 |
| Total cholesterol (mg/dL) | 112 | 181.4±32.1 | 33 | 171.7±29.5 |
| HDL-C (mg/dL) | 108 | 48.6±14.7 | 31 | 44.1±10.5 |
| LDL-C (mg/dL) | 108 | 107.1±26.5 | 31 | 105.9±28.9 |
| Triglycerides (mg/dL) | 111 | 159.6±93.7 | 33 | 144.6±73.7 |
| Uric acid (mg/dL) | 112 | 5.11±1.40 | 33 | 4.85±1.58 |
| BUN (mg/dL) | 115 | 16.3±10.7 | 33 | 16.7±18.6 |
| Creatinine (mg/dL) | 114 | 0.74±0.23 | 32 | 0.65±0.13 |
| Hematocrit (%) | 114 | 42.5±4.4 | 32 | 43.6±3.8 |
| Hemoglobin (g/dL) | 114 | 14.4±1.6 | 32 | 14.9±1.4 |
Data are expressed as mean ± standard deviation or, number and percentage of the patients.
n: the number of patients evaluated.
Concomitant antidiabetic drugs used in the study period
An SGLT2 inhibitor, dapagliflozin, was used as a monotherapy during the study period in 9.4% of the total patient cohortand in 3.0%of patients with fatty liver. Other patients were treated with concomitant antidiabetic drugs, and DPP-4 inhibitor, sulfonylurea, biguanide, insulin and GLP-1 receptor agonistwere commonly used (10% or more) in both patient groups.Especially, many patients with fatty liver were treated with DPP-4 inhibitor (78.8%) or biguanide (30.3%)(Table 2).
Table 2. Concomitant antidiabetic drugs used in the study period.
| Concomitant antidiabetic drugs n [%] | Total (N=117) | With fatty liver (N=33) |
|---|---|---|
| None | 11 [9.4] | 1 [3.0] |
| DPP-4 inhibitor | 65 [55.6] | 26 [78.8] |
| Sulfonylurea | 26 [22.2] | 6 [18.2] |
| Biguanide | 29 [24.8] | 10 [30.3] |
| Insulin | 20 [17.1] | 6 [18.2] |
| GLP-1 receptor agonist | 13 [11.1] | 5 [15.2] |
| Thiazolidine | 7 [6.0] | 0 |
| Glinide/α-GI | 5 [4.3] | 1 [3.0] |
| α-GI | 6 [5.1] | 2 [6.1] |
| Glinide | 2 [1.7] | 1 [3.0] |
| DPP-4 inhibitor/thiazolidine | 2 [1.7] | 0 |
Data are expressed as number and percentage of the patients.
Changes in HbA1c and blood glucose
The mean HbA1c was 7.52 ± 1.23% at baseline and significantly (p<0.001) reduced to7.20 ± 1.15% at 12 months after treatment, with a reduction of -0.32% (-4.3% reduction) in the total patientcohort. The mean HbA1c was 7.61 ± 1.35% at baseline and significantly (p<0.01) reduced to 7.11 ± 1.13% at 12 months after treatment, with a reduction of -0.50% (-6.6% reduction) in patients with fatty liver (Figure 1).
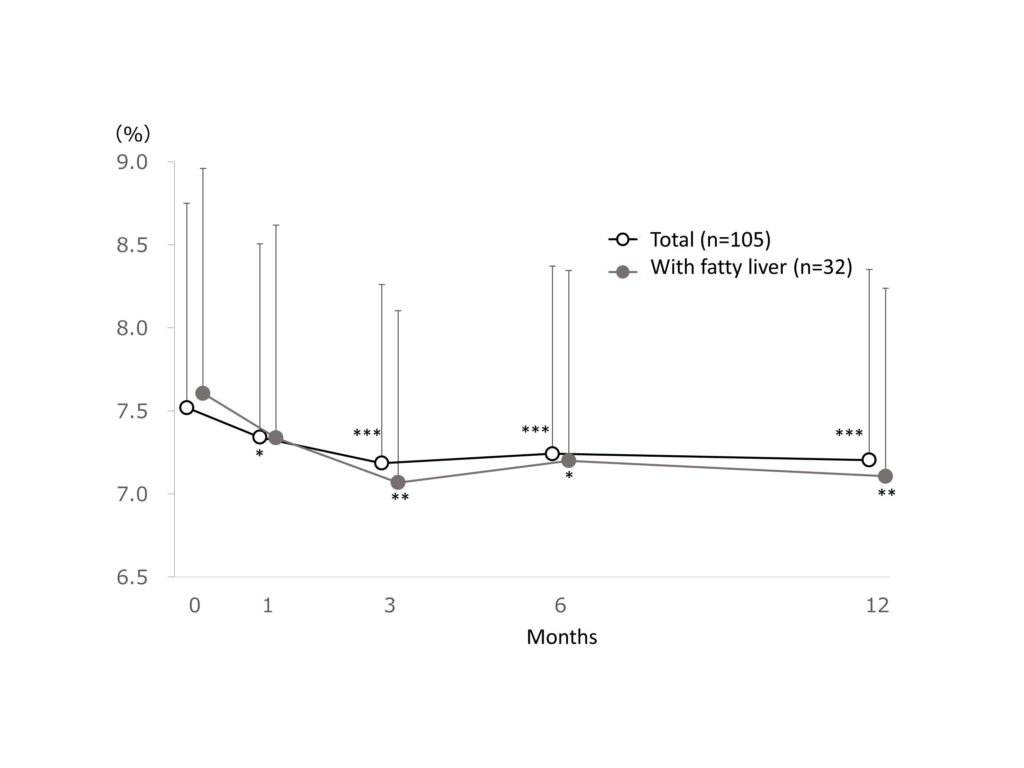
Figure 1.Change in HbA1c.
Change in HbA1c. Data are expressed as mean + standard deviation. The missing data were imputed using last observation carried forward. The ANOVA test was used for statistical analysis, adjusting the multiplicity with the Holm method (*: p<0.05, **: p<0.01, ***: p<0.001 vs baseline).
The mean blood glucose was 171.3 ± 61.4 mg/dL at baseline and significantly (p<0.01) reduced by -20.6 mg/dL (-12.0% reduction) at 12 months after treatment in the total patient cohort. The mean blood glucose was 164.0 ± 48.9 mg/dL at baseline and reduced by -15.7 mg/dL (-9.5% reduction) at 12 months after treatment in patients with fatty liver (Figure2).
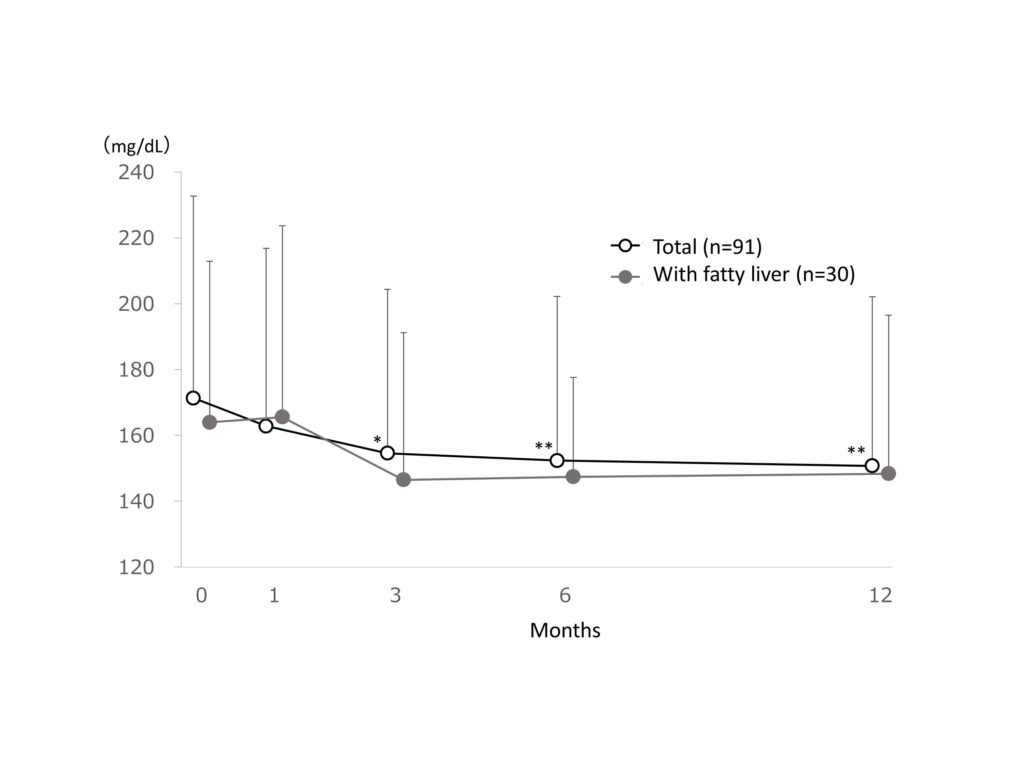
Figure 2.Change in blood glucose
Change in blood glucose. Data are expressed as mean + standard deviation. The missing data were imputed using last observation carried forward. The ANOVA test was used for statistical analysis, adjusting the multiplicity with the Holm method (*: p<0.05, **: p<0.01 vs baseline).
Change in BMI
The mean BMI was 29.6 ± 5.2 kg/m2 at baseline and significantly (p<0.001) reduced to28.6 ± 5.0 kg/m2 at 12 months after treatment, with a reduction of -1.0 kg/m2(-3.5% reduction) in the total patient cohort. The mean BMI was 29.3 ± 5.2 kg/m2 at baseline and significantly (p<0.01) reduced to28.2 ± 5.1 kg/m2 at 12 months after treatment, with a reduction of -1.1kg/m2(-3.8% reduction) in patients with fatty liver (Figure 3).
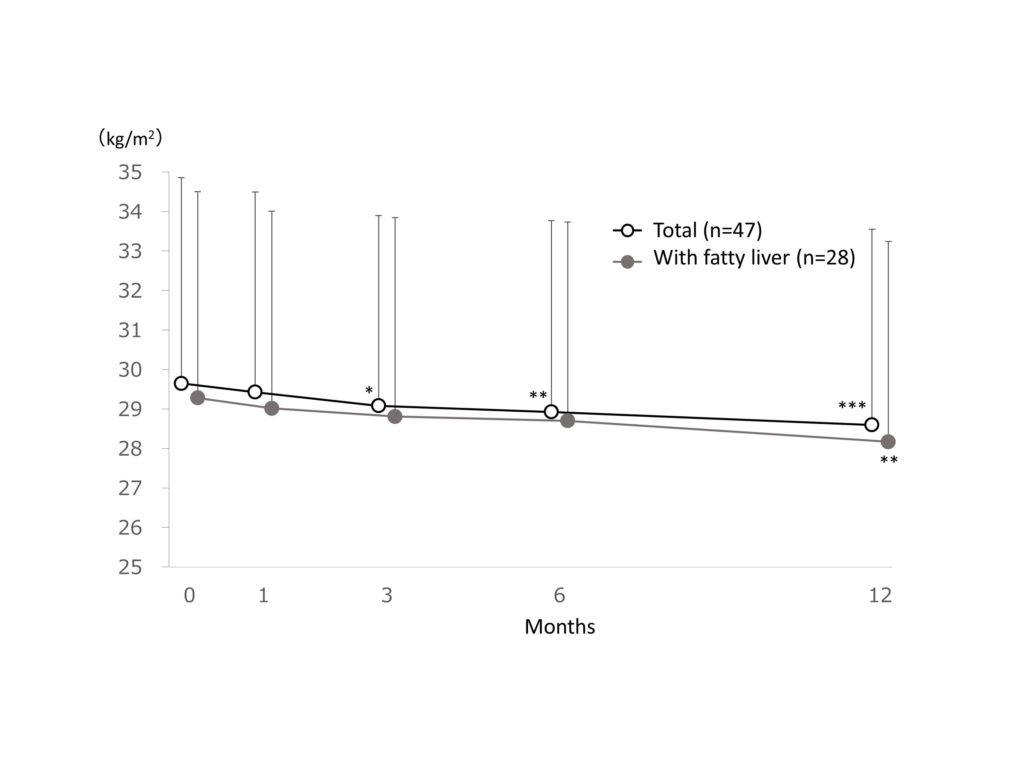
Figure 3.Change in BMI.
Change in BMI. Data are expressed as mean + standard deviation. The missing data were imputed using last observation carried forward. The ANOVA test was used for statistical analysis, adjusting the multiplicity with the Holm method (*: p<0.05, **: p<0.01, ***: p<0.001 vs baseline).
Changes in liver function parameters
Changes in AST, ALT, and γ-GTP are shown in Figure 4A, B and C, respectively. The mean AST was 41.6 ± 41.3IU/L at baseline and significantly (p<0.001) reduced to 29.7 ± 14.6IU/L at 12 months after treatment, with a reduction of -11.9IU/L (-28.7% reduction) in the total patient cohort. The mean AST was 59.7 ± 41.6IU/L at baseline and significantly (p<0.001) reduced to 38.4 ± 17.1IU/L at 12 months after treatment, with a reduction of -21.3IU/L (-35.7% reduction) in patients with fatty liver (Figure 4A). The mean ALT was 49.0 ± 57.5 IU/L at baseline and significantly (p<0.001) reduced to 34.8 ± 27.1 IU/L at 12 months after treatment, with a reduction of -14.2 IU/L (-29.0% reduction) in the total patient cohort. The mean ALT was 81.8 ± 82.5 IU/L at baseline and significantly (p<0.001) reduced to 52.1 ± 37.1 IU/L at 12 months after treatment, with a reduction of -29.7 IU/L (-36.3% reduction) in patients with fatty liver (Figure 4B). The mean γ-GTP was 64.7 ± 106.1 IU/L at baseline and significantly (p<0.05) reduced to 55.3 ± 97.7 IU/L at 12 months after treatment, with a reduction of -9.4 IU/L (-14.6% reduction) in the total patient cohort. The mean γ-GTP was 75.7 ± 40.8 IU/L at baseline and reduced to 67.7 ± 75.1 IU/L at 12 months after treatment, with a reduction of -8.0 IU/L (-10.6% reduction) in patients with fatty liver (Figure 4C).
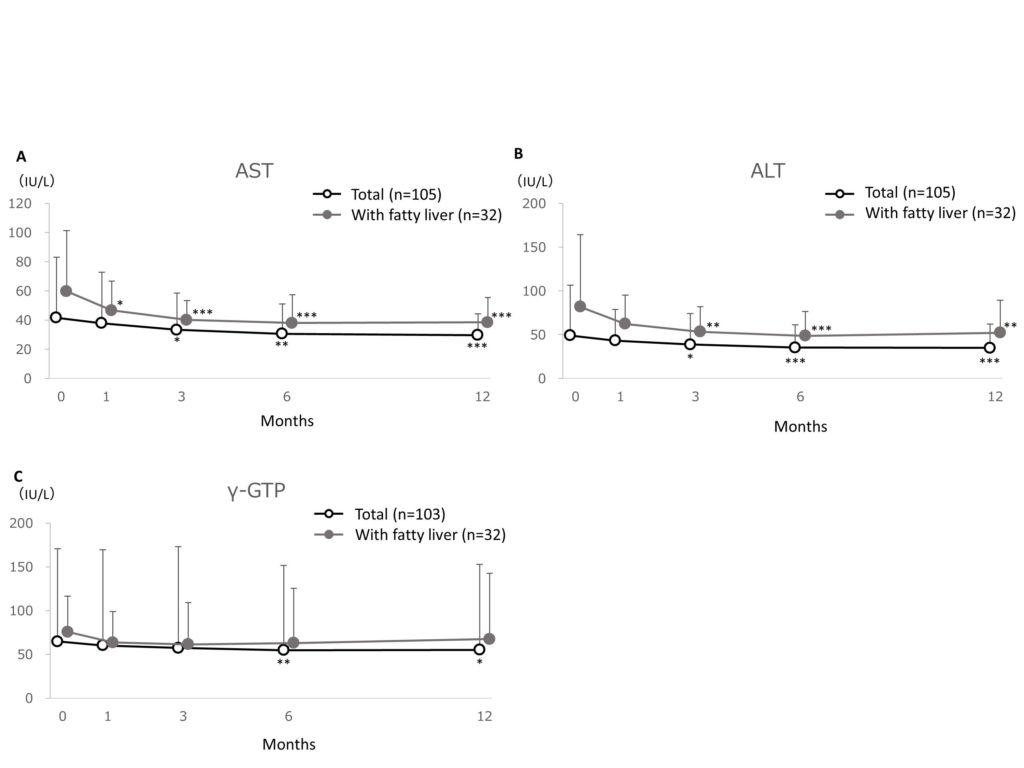
Figure 4.Changes in liver function parameters, AST (A), ALT (B) and γ-GTP (C).
Changes in liver function parameters, AST (A), ALT (B) and γ-GTP (C). Data are expressed as mean + standard deviation. The missing data were imputed using last observation carried forward. The ANOVA test was used for statistical analysis, adjusting the multiplicity with the Holm method (*: p<0.05, **: p<0.01, ***: p<0.001 vs baseline).
Changes in liver stiffness and steatosis evaluated by transient elastography
Before and after dapagliflozintreatment, the liver stiffness and steatosis were evaluated using the transient elastography(FibroScan®) based on the index of E and CAP values, respectively. The E and CAP values varied widely among patients, and no obvious change was totally observed after treatment with dapagliflozin in both patient groups (Figure 5A and B).

Figure 5.Changes in liver stiffness (A) and steatosis (B) evaluated by transient elastography
Changes in liver stiffness (A) and steatosis (B) evaluated by transient elastography. The FibroScan® was used for the evaluation, and the measurement data were obtained as E value for the index of liver stiffness and controlled attenuation parameter (CAP) value for the index of liver steatosis. The figures show the dot-plots of each measured value obtained before and after the treatment of dapagliflozin, and the data are expressed as mean ± standard deviation. The paired t-test was used for statistical analysis between the values of before (pre) and after (post) the treatment (p>0.05 in all analyses).
Case report
Figure 6 shows the case report for the changes in HbA1c, ALT, AST, γ-GTP, liver stiffness (E value), and liver steatosis (CAP value) evaluated by transient elastography during the 12-month study period. This patient was male, aged 69 years,had fatty liver, and could be continuously evaluated for these parameters for12 months in the clinical setting.These parameters were likely to improve parallel in this patient.
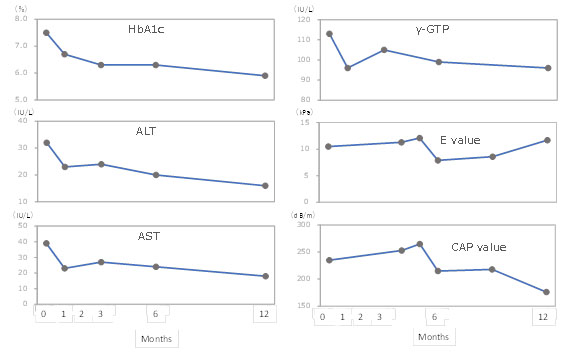
Changes in laboratory test parameters
The HDL-C, BUN, hematocrit and hemoglobin were significantly increased in both patient groups (p<0.01). Uric acid was significantly reduced in the total patient cohort (p<0.05), and tended to be reduced in patients with fatty liver (Table 3).
Table 3. Changes in laboratory test parameters
| Total (N=117) | With fatty liver (N=33) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Pre | Post | P value | n | Pre | Post | P value | |||
| Total cholesterol
(mg/dL) |
103 | 181.2
±33.0 |
185.0
±33.3 |
0.161 | 32 | 170.8
±29.6 |
176.4
±33.5 |
0.317 | ||
| HDL-C
(mg/dL) |
99 | 48.6
±14.7 |
51.2
±15.1 |
<0.001*** | 29 | 43.9
±9.7 |
47.1
±11.0 |
0.004** | ||
| LDL-C
(mg/dL) |
99 | 107.2
±27.1 |
107.2
±29.0 |
0.990 | 29 | 106.9
±29.2 |
105.1
±35.2 |
0.733 | ||
| Triglycerides
(mg/dL) |
103 | 162.5
±95.8 |
158.8
±97.4 |
0.636 | 32 | 146.5
±74.0 |
147.7
±92.6 |
0.891 | ||
| Uric acid
(mg/dL) |
105 | 5.11
±1.39 |
4.90
±1.25 |
0.045* | 32 | 4.92
±1.56 |
4.67
±1.27 |
0.221 | ||
| BUN
(mg/dL) |
104 | 16.3
±11.1 |
17.4
±11.3 |
0.002** | 32 | 16.9
±18.9 |
18.6
±19.6 |
0.006** | ||
| Creatinine
(mg/dL) |
103 | 0.74
±0.21 |
0.75
±0.21 |
0.137 | 31 | 0.65
±0.13 |
0.66
±0.14 |
0.350 | ||
| Hematocrit
(%) |
103 | 42.4
±4.4 |
44.5
±4.3 |
<0.001*** | 31 | 43.6
±3.9 |
45.8
±3.3 |
<0.001*** | ||
| Hemoglobin
(g/dL) |
103 | 14.4
±1.6 |
15.0
±1.6 |
<0.001*** | 31 | 14.9
±1.4 |
15.6
±1.2 |
<0.001*** | ||
Data are expressed as mean ± standard deviation. n: the number of patients evaluated. The paired t-test was used for statistical analysis between the values of before (pre) and after (post) the treatment (*: p<0.05, **: p<0.01, ***: p<0.001).
Adverse events
No clinically significant adverse events(AE) and abnormal laboratory test results were observed during the study period.
Discussion
This study was conducted in actual clinical setting, to investigate the influences of SGLT2 inhibitor, dapagliflozin, on blood glucose, BMI, liver function parameters, liver stiffness and steatosis evaluated by transient elastography, and laboratory test parameters among Japanese T2DM outpatientswith fatty liver.
Compared with the total patient cohort, patients with fatty liver had higher baseline values of AST, ALT, and γ–GTP,suggesting the reduction of liver function in these patients.Obesity was observed in both patient groups, with a mean BMI of 29.3 and 29.2 kg/m2in the total patient cohort and patients with fatty liver, respectively. The proportion of patients with fatty liver treated with dapagliflozin monotherapy was smaller than the proportion of the total patient cohort treated with dapagliflozin monotherapy (3.0% vs. 9.4%), suggesting that more intensive therapy for T2DM was needed in patients with fatty liver and obesity, because these disorders may serve as a progression factor for the other[6].
In particular, a large proportion ofpatients with fatty liver was concomitantlytreated with a DPP-4 inhibitor (78.8%). This may be because DPP-4 inhibitorsare thought to have a neutral effect on body weight[27], and were more likely used as concomitant drugs for T2DM patients with fatty liver and obesity.
The mean HbA1c was 7.61% at baseline and significantly reduced to 7.11% at 12 months after treatment, with a reduction of -0.50% in patients with fatty liver.Most of these patients (78.8%) were concomitantly treated with a DPP-4 inhibitor during the study period. A previous randomized controlled phase 3 study that assessedthe efficacy of dapagliflozin as add-on therapy to the DPP-4 inhibitor, sitagliptin, in patients with T2DM(not necessarily with fatty liver) demonstrated that the reduction inHbA1c was -0.5% (baseline HbA1c: 7.9%) at 24 weeks after treatment[28]. These results suggest that the hypoglycemic efficacy of dapagliflozin issimilar amongT2DM patients with and without fatty liver.
The mean BMI significantly reduced from 29.3 kg/m2 at baseline to 28.2 kg/m2 at 12 months after treatment with dapagliflozin in patients with fatty liver. A systematic review and meta-analysis of randomized trials indicates that ≥5% weight loss improved hepatic steatosis, and ≥7% weight loss also improved the NAFLD activity score[29], therefore weight loss is thought to be an important therapeutic strategy in patients with NAFLD. Dapagliflozin is suggested to be useful in improving the conditions of NAFLD associated with T2DM.
In the present study, liver function parameters such as AST and ALT were significantly reduced after dapagliflozin treatment in patients with fatty liver. The mean AST was 59.7 IU/L at baseline and significantly reduced by -21.3 IU/L (-35.7% reduction) at 12 months after treatment, and the mean ALT was 81.8 IU/L at baseline and significantly reduced by -29.7 IU/L (-36.3% reduction). Recently, the effects of dapagliflozin on AST and ALT were evaluated among T2DM patients with nonalcoholic steatohepatitis (NASH)(n=11) in a prospective, open-label, uncontrolled study. In that study, the median AST was 52 IU/L at baseline and was significantly reduced by -26 IU/L(-50.0% reduction) at 24 weeks after treatment, and the median ALT was 59 IU/L at baseline and significantly reduced by -29 IU/L (-49.2% reduction)[21]. The absolute amounts of AST and ALT reductions cannot be directly compared between these twostudies because of the differencesin the mean and median values in the respective study; however, dapagliflozin is thought toimprove the liver function in T2DM patients with fatty liver or NASH.
Growing evidence suggests that NAFLD is associated with an increased risk of cardiovascular disease beyond that conferred by established risk factors[30], and it is reported that NAFLD increases the risk to develop also serious hepatic diseases in particular NASH, cirrhosis and hepatocellular carcinoma[7], which possibly read to reduced life expectancy in patients with T2DM[31,32].The SGLT2 inhibitor, dapagliflozin, is suggested to improve not only hyperglycemia but also deterioration of liver conditions in T2DM patients with NAFLD and is expected to become a useful therapeutic option in these patients.
Transient elastography using FibroScan®, which measures liver stiffness or steatosis, is a novel, noninvasive method to assess liver fibrosis[25,26]. It has been reported that a significant correlation between liver stiffness measurement using FibroScan® and fibrosis stage in patients with NAFLD, as confirmed by the results of liver biopsy in patients with NASH[33]. This measurement is also demonstrated to be an effective procedure to screen for fibrosis and steatosis in DM patients[34,35].However, there have been few reports of the evaluation for liver stiffness and steatosis after the pharmacological treatment of T2DM in patients with fatty liver, such asNAFLD or NASH. Therefore, in the present study, liver stiffness and steatosis were evaluated using this transient elastography in T2DM patients with fatty liver, who were treated with SGLT2 inhibitor, dapagliflozin. The E and CAP values varied widely among T2DM patients with fatty liver in the present study, and no obvious change was observed in these parameters after the treatment with dapagliflozin,although liver function parameters were clearly improved. As one of the reason for this result, we believe that the evaluation period was short; almost half(43%) of the T2DM patients with fatty liverwere evaluated using these parameters within 6 months after treatment, because the transient elastography was performed as part of the daily clinical practice, and we did not set any specific and favorable timing for evaluation in this study.
A case report for one patient who could be regularly monitored until12 months after the dapagliflozin treatment indicated that changesin E and CAP values were associated with those of HbA1c and liver function parameters. Several SGLT2 inhibitors, including dapagliflozin, have been shown benefit in animal models of NAFLD[17,18], and have been demonstrated improved liver conditions such as reducing fatty liver index score in some clinical studies with T2DM patients[19-23]. Considering together, the E and CAP valuesmeasured usingFibroScan®may becomeuseful in examining the changesin liver stiffness and steatosis in T2DM patients with fatty liver treated with SGLT2 inhibitors. Recently, fibrosis markers for NAFLD/NASH, such as hyaluronic acid and type IV collagen,have been studied and developed[36,37]. The relations of these markers and the transient elastography data obtained from T2DM patients with fatty liver treated with SGLT2 inhibitor are interesting, and expected to be investigated in the near future.
Changes in laboratory test parameters were generally consistent with the previous reports on dapagliflozin treatment in T2DM patients[38], and no clinically significant AEs including notably abnormal laboratory tests were observed in the study period. Dapagliflozin maybe used safely in T2DM patients with fatty liver in the clinical setting.
The present study first evaluated the influences of the SGLT2 inhibitor on liver stiffness and steatosis evaluated usingtransient elastography, in addition to liver function parameters, among Japanese T2DM patients with fatty liver in the clinical setting. However, the number of study patients was small, and the study had no control groupcontaining patients who were not treated with dapagliflozin.Further investigations are needed to validate the findings obtained in the present study, especially to explore the usefulness of transient elastography in T2DM patients with fatty liver treated with SGLT2 inhibitors.
In clinical setting, dapagliflozin improved HbA1c, reducing BMI and improving liver function parameters such as AST and ALTin Japanese T2DM patients with fatty liver. Transient elastography could be a useful method for evaluating the influence of liver stiffness and steatosis in T2DM patients with fatty liver treated with SGLT2 inhibitors.
Acknowledgements
Data analysis and medical writing supports were provided by Interscience Co., Ltd. with the fund from Ono Pharmaceutical Co., Ltd. and AstraZeneca K.K.
References
Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol.2018; 14: 88-98.
Holman N, Young B, Gadsby R. Current prevalence of Type 1 and Type 2 diabetes in adults and children in the UK. Diabet Med. 2015; 32: 1119-20.
Bruno G, Runzo C, Cavallo-Perin P, et al. Incidence of type 1 and type 2 diabetes in adults aged 30-49 years: the population-based registry in the province of Turin, Italy. Diab Care.2005; 28: 2613-9.
Loomba R, Sanyal AJ. The global NAFLD epidemic.Nat Rev GastroenterolHepatol. 2013; 10: 686-90.
Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA.2015; 313: 2263-73.
Williams KH, Shackel NA, Gorrell MD, et al. Diabetes and nonalcoholic Fatty liver disease: a pathogenic duo. Endocr Rev. 2013; 34: 84-129.
Radaelli MG, Martucci F, Perra S, et al. NAFLD/NASH in patients with type 2 diabetes and related treatment options. J Endocrinol Invest. 2017.
Bril F, Cusi K. Management of Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes: A Call to Action. DiabCare.2017; 40: 419-30.
Thrasher J. Pharmacologic Management of Type 2 Diabetes Mellitus: Available Therapies. Am J Cardiol. 2017; 120(Suppl): S4-S16.
Jung CH, Jang JE, Park JY. A Novel Therapeutic Agent for Type 2 Diabetes Mellitus: SGLT2 Inhibitor. Diabetes Metab J. 2014; 38: 261-73.
Goring S, Hawkins N, Wygant G, et al. Dapagliflozin compared with other oral anti-diabetes treatments when added to metformin monotherapy: a systematic review and network meta-analysis. Diabetes ObesMetab. 2014; 16: 433-42.
Liu XY, Zhang N, Chen R, et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2years. J Diab Complications.2015; 29: 1295-303.
Zaccardi F, Webb DR, Htike ZZ, et al. Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: systematic review and network meta-analysis. Diabetes ObesMetab. 2016; 18: 783-94.
Shyangdan DS, Uthman OA, Waugh N. SGLT-2 receptor inhibitors for treating patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. BMJ Open. 2016; 6: e009417.
Heerspink HJ, Perkins BA, Fitchett DH, et al. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation. 2016; 134: 752-72.
Lytvyn Y, Bjornstad P, Udell JA, et al. Sodium Glucose Cotransporter-2 Inhibition in Heart Failure: Potential Mechanisms, Clinical Applications, and Summary of Clinical Trials. Circulation. 2017; 136: 1643-58.
Hayashizaki-Someya Y, Kurosaki E, Takasu T, et al. Ipragliflozin, an SGLT2 inhibitor, exhibits a prophylactic effect on hepatic steatosis and fibrosis induced by choline-deficient l-amino acid-defined diet in rats. Eur J Pharmacol. 2015; 754: 19-24.
Qiang S, Nakatsu Y, Seno Y, et al. Treatment with the SGLT2 inhibitor luseogliflozin improves nonalcoholic steatohepatitis in a rodent model with diabetes mellitus. DiabetolMetabSyndr. 2015; 7: 104.
Katsuyama H, Hamasaki H, Adachi H, et al. Effects of Sodium-Glucose Cotransporter 2 Inhibitors on Metabolic Parameters in Patients With Type 2 Diabetes: A Chart-Based Analysis. J Clin Med Res. 2016; 8: 237-43.
Hayashi T, Fukui T, Nakanishi N, et al. Dapagliflozin decreases small dense low-density lipoprotein-cholesterol and increases high-density lipoprotein 2-cholesterol in patients with type 2 diabetes: comparison with sitagliptin. CardiovascDiabetol. 2017; 16: 8.
Tobita H, Sato S, Miyake T, et al. Effects of Dapagliflozin on Body Composition and Liver Tests in Patients with Nonalcoholic Steatohepatitis Associated with Type 2 Diabetes Mellitus: A Prospective, Open-label, Uncontrolled Study. CurrTher Res Clin Exp. 2017; 87:13-9.
Takase T, Nakamura A, Miyoshi H, et al. Amelioration of fatty liver index in patients with type 2 diabetes on ipragliflozin: an association with glucose-lowering effects. Endocr J. 2017; 64: 363-7.
Seko Y, Sumida Y, Tanaka S, et al. Effect of sodium glucose cotransporter 2 inhibitor on liver function tests in Japanese patients with non-alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol Res. 2017; 47: 1072-8.
The Japan Diabetes Society. The practice guideline for the treatment for diabetes in Japan 2016 (in Japanese).
Wong VW, Vergniol J, Wong GL, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology.2010; 51: 454-62.
Sasso M, Beaugrand M, deLedinghen V, et al. Controlled attenuation parameter (CAP): a novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010; 36: 1825-35.
American Diabetes Association. Obesity Management for the Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018; 41: S65-S72.
Jabbour SA, Hardy E, Sugg J, et al. Dapagliflozin is effective as add-on therapy to sitagliptin with or without metformin: a 24-week, multicenter, randomized, double-blind, placebo-controlled study. Diabetes Care.2014; 37: 740-50.
Musso G, Cassader M, Rosina F, et al. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia.2012; 55: 885-904.
Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010; 363: 1341-50.
Trombetta M, Spiazzi G, Zoppini G, et al. Review article: type 2 diabetes and chronic liver disease in the Verona diabetes study. Aliment PharmacolTher. 2005; 22(Suppl): 24-7.
De Marco R, Locatelli F, Zoppini G, et al. Cause-specific mortality in type 2 diabetes. The Verona Diabetes Study.Diabetes Care.1999; 22: 756-61.
Yoneda M, Yoneda M, Mawatari H, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with nonalcoholic fatty liver disease (NAFLD). Dig Liver Dis. 2008; 40: 371-8.
Koehler EM, Plompen EP, Schouten JN, et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: The Rotterdam study. Hepatol.2016; 63: 138-47.
Kwok R, Choi KC, Wong GL, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut.2016; 65: 1359-68.
Dvorak K, Stritesky J, Petrtyl J, et al. Use of non-invasive parameters of non-alcoholic steatohepatitis and liver fibrosis in daily practice–an exploratory case-control study. PLoS One. 2014; 9: e111551.
Mizuno M, Shima T, Oya H, et al. Classification of patients with non-alcoholic fatty liver disease using rapid immunoassay of serum type IV collagen compared with liver histology and other fibrosis markers. Hepatol Res. 2017; 47: 216-25.
Ptaszynska A, Hardy E, Johnsson E, et al. Effects of dapagliflozin on cardiovascular risk factors. Postgrad Med. 2013; 125: 181-9.
Received: October 02, 2018;
Accepted: October 20, 2018;
Published: October 30, 2018.
To cite this article : Takaguchi K, Tsutsui A, Senoo T, et al. Effects of dapagliflozin in type 2 diabetes patients with fatty liver. Japan Journal of Medicine. 2018: 2:1.
©Takaguchi K, et al. 2018.
