Review Article / Open Access
DOI: 10.31488/jjm.1000119
Kidney development and susceptibility to develop kidney disease in adulthood
Faa G1, Fanni D*1, Gerosa C1, Gibo Y2, Fanos V3
*Corresponding author:Dr Daniela Fanni, MD, PhD, Department of Pathology, University of Cagliari, “S. Giovanni di Dio” University Hospital, via Ospedale 54, 09124 Cagliari, Italy. Tel: 0390706092372;Fax 0396092115
Abstract
Human nephrogenesis is physiologically completed between the 35th and the 36th week of gestation. Pre-term infants may continue nephrogenesis after birth only for 2-4 weeks. Extreme immaturity at birth may be associated to impaired nephrogenesis and with oligonephronia. In this minireview, the hypothesis that low birth weight, preterm delivery and perinatal nutrient restriction could increase the susceptibility to develop renal disease in adulthood is discussed. Moreover, we consider a new approach to the prevention of chronic kidney disease in adulthood, based on regenerative prolonging nephrogenesis until 36 weeks in all preterm infants.
Keywords: nephrogenesis, kidney development, chronic kidney disease,fetal programming
Main Text
The development of the kidney is a complex process that requires interactions among a high number of pluripotential stem/progenitor cells, including metanephricmesenchimal cells and epithelial progenitors originating from the ureteric bud[1]. The coordinate renal development during the intrauterine life leads to the coordinate differentiation of multiple specialized cell types that characterize the mature kidney architecture [2]. Nephrogenesis, i.e. glomerulogenesis and tubulogenesis, occurs in the fetal kidney in the subcapsular zone, where mesenchymal progenitors addensate, giving rise to a dark area called “blue strip”, whose depth may beutilized for evaluating the residual nephrogenic potential of a neonatal kidney (Figure 1)[3].
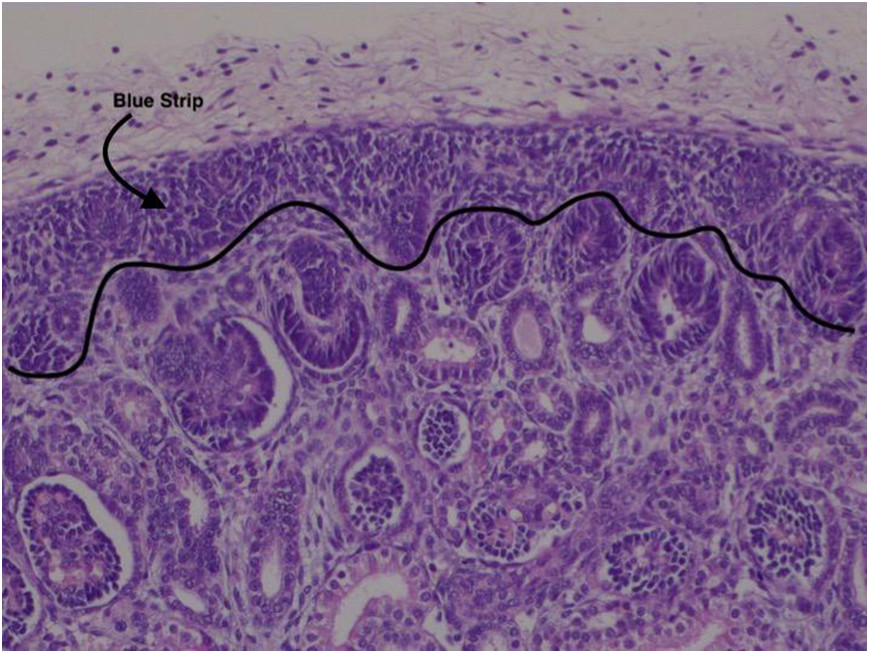
Figure 1.Area of nephrogenesis: blue strip.
Glomerulogenesis depends on the interaction between two components characterized by a different developmental history: the branching epithelial tubules originating from the ureteric bud, and the metanephric mesenchymal cells [4]. The cells of the tips of the epithelial branches, once arrived in close proximity to the renal capsule, take in contact with the mesenchymal precursors, giving rise to the cap mesenchymal cells, characterised by the strong expression of Bcl-2, an anti-apoptotic protein[5]. The interaction between the cells of the epithelial ampullae and the cap mesenchyme induces the mesenchymal –epithelial transition of mesenchymal progenitors, giving rise to renal vesicles, each of which originates a new nephron[6]. At the same time, different pools of metanephric mesenchymal stem cells differentiate into interstitial progenitors that originate the capsular, cortical, medullary and hilar interstitial stromal cells[7]. Given this complexity, the existence of multiple stem cell niches in different compartments of the developing kidney has beenhypothesized, each of them being involved in kidney development bycoordinating the development of different renal cell types[8].
Human nephrogenesis is characterised by two principal aspects that play a relevant role in clinical practice. The former is represented by the assumption that nephrogenesis is physiologically completed before birth, between the 35th and the 36th week of gestation. This means that our ability to generate new nephrons is absent in the postnatal life, so that the number of nephrons we are born with will represent our nephron burden along the whole life. The latter is the ability of pre-term infants, born before 36 weeks of gestation, to continue renal maturation and nephrogenesis after birth, but only for 2-4 weeks[9]. This means that a preterm baby, born at 25 weeks, will continue to form new nephrons till the 28th or the 29th post-conceptional week(Figure 2), loosing at least 7 weeks of nephrogenesis and becoming a oligonephronic individual [10]. In brief, extreme immaturity at birth has been associated, in recent years, with impaired nephrogenesis and with oligonephronia in childhood and in adulthood. Marked disparities in nephron number have been reported not to be restricted to immaturity, being related to multiple causes, including low birth weight [11]and perinatal nutrient restriction [12]. A marked inter-individual variability in kidney maturation and in nephron number at birth, independent from gestational age, has been reported by our group in human preterm and at term newborns [2], confirming previous hypotheses that the variability of nephron number might represent, in humans, a rule[13].The average nephron number in humans has been estimated to be approximately 900,000 to 1 million per kidney, but it probably rangesfrom 300,000 up to 1,800,000 per kidney[14]. According withother authors, the number of glomeruli for individual kidneys might range from approximately 200,000 to 2.5 million, i.e. it could change more that ten times from one individual to the next [15]. Considering two subjects at the extremes of the spectrum, one carrying 200,000 and the other 2,5 million glomeruli per kidney, the clinical significance of a pathological event occurring in adult life and destroying 100,000 nephrons could be completely different. According with the Brenner’s hypothesis on fetal andperinatal programming of adult diseases, low birth weight, preterm delivery and perinatal nutrient restriction have been identified as possible causes of a reduced nephron number, a factor able to increase the susceptibility to renal disease in adulthood [12,16]. In brief, low birth weight and low nephron number at birth has become, in recent years, a major factor of clinical relevance for nephrologists, being associated with the increasing risk for chronic kidney disease in adulthood [17]. Whereas the vast majority of studies on the perinatal programming of kidney diseases were focused on the analysis of the multiple factors involved in intrauterine growth restriction and on the causes of low nephron number[18], some researchers started to analyse the mechanisms able to accelerate nephrogenesis in the perinatal period [19]. Given that nephrogenesis in preterm babies may continue for 2-4 weeks after birth [9]our group hypothesized a new approach to the prevention of hypertension and chronic kidney disease in adulthood, to be started in the perinatal period. We defined this new approach as “physiological” renal regenerating medicine, being mainly based on prolonging nephrogenesis until 36weeks of post conceptual age in all preterm infants. The goal of this new approach might be to allow newborn kidneys to restore theirnephron endowment and to escape oligonephronia and chronic kidney disease later in life [20].The basis of this hypothesis, suggesting the necessity of starting a renal regenerative medicine in the perinatal period, is mainly based on the structural differences of the kidney in preterm babies. The preterm kidney is characterized by the presence of a huge amount of active endogenous stem/progenitor cells, whose complexity has been clarified only in recent times with immunohistochemical analyses [5, 21]. In our opinion, these renal progenitor/stem cells, that are physiologically abundant in the kidney of preterm babies, represent a unique opportunity for preventing oligonephronia. The knowledge of the multiple markers expressed by mesenchymal stem cells might allow neonatologists to induce mesenchymal-to-epithelial transition and glomerulogenesis even after birth, changing the fate of renal stem cells and restoring the nephron burden that could be markedly low due topreterm delivery[22]. These are the bases of a new approach prospected, in recent years, by our group: the opportunity to start regenerative medicine in preterm and low birth weight infants in the perinatal period, by inducing pluripotent stem cells of the kidney to generate new nephrons after birth, improving kidney function and protecting them from developing chronic kidney disease and end stage renal disease later in childhood as well as in adulthood[23]. The vast majority of studies on kidney regeneration medicine were mainly focused on adult patients affected by chronic kidney diseasewith the aim to create a new stem cell-based approach to treat patients affected by renal failure, given the limitations of dialysis and kidney transplant to solve the problem [24]. Unfortunately, such regenerative approaches have been halted by the completedisarrangement of the renal architecture due to nephron loss and interstitial fibrosis[25]. Even the most recent approaches, based on the use of a kidney scaffold and on its recellularizationutilising renal stem/progenitor cells, have encountered many obstacles, and this fascinating strategy for renal regenerative medicine is under evaluation by the scientific community[26].
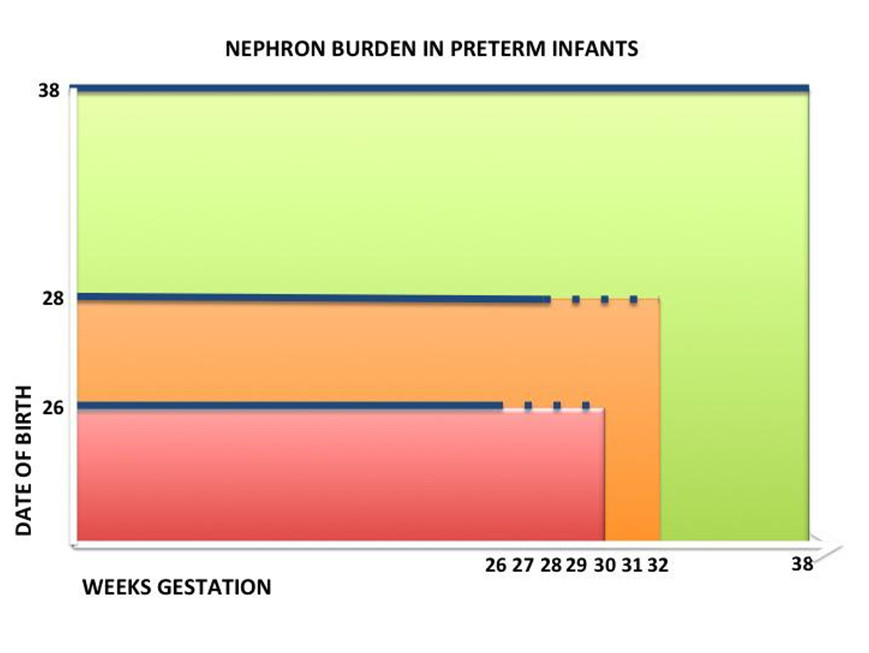
Figure 2.Schematicdiagram of the evolutionnephron in preterm.
Our proposal starts from an “embryological” view of renal disease: our susceptibility to develop chronic kidney disease and, eventually renal failure in adulthood starts in utero, and it is mainly due to an abnormal programming of kidney development, ending with a low number of functioning glomeruli[27].Our nephron burden is highly conditioned by the intrauterine environment and by preterm delivery, nephron number being related to the weeks of gestation at birth, and to body weight at birth [2,28]. Moreover, events like maternal malnutrition, maternal diabetes, infections, maternal drug exposure and perinatal asphyxia may cause intrauterine growth restriction and sustain reduction of functioning glomeruliwith hyper-filtration of the remaining ones, predisposing to individual susceptibility to chronic kidney disease later in life [29]. Our hypothesis is that individual who are born with fewer functioning glomeruli should be predicted to develop renal disease later in life, when a second “hit”, represented by any nephrotoxic agent, would destroy a significant number of nephrons, modifying the ability of the remaining glomeruli to assure a proper renal function. In brief, it appears plausible that a kidney with an abundant number of glomeruli (for example: 2 million per kidney) might be able to withstand the multiple injuries that may occur in the lifespan, without significant consequences on the global renal function. At the otherextreme of the spectrum, it is plausible that a kidney with ten fold less glomeruli (for example: 200,000 per kidney), allowing a sufficient renal function in physiological conditions, will be less able to counteract additional injuries that may occur later in life, ending with renal insufficiency. From a practical point of view, even though data are not robust enough at the moment to certainly correlate low birth weight and preterm birth with end stage kidney disease [30], all these findings taken together appear intriguing and of high potential interest.
Differently from other approaches of regenerative medicine, mainly based on the use of renal adult exogenous stem cells and focused on adult patients, our opinion is that renal regenerative medicine should develop new methodologies, stemmed from the previous attempts. Our data on low birth weight newborns have confirmed that they are characterized by a reduced nephron number at birth and, as a consequence, they represent a population at increased risk of developing chronic kidney disease in childhood or in adulthood [10].
Given that the newborn kidney, particularly in the preterm infants, is characterized by a huge number of active stem/precursor cells, the perinatal period represents a fascinating window, for a preventive regenerative approach to chronic kidney disease. This regenerative proposal contains two main innovative factors, as compared to the classical regenerative approaches. It should be mainly based on endogenous stem cells, and in particular on the metanephric mesenchymal cells that are physiologically present, in huge amounts, in the preterm kidney [31].The second peculiar aspect of our proposal is the preventive approach, to be localized in the perinatal period, starting immediately after birth, and managed by neonatologists [31]. The timingof our regenerative proposal is mandatory, depending on the very short survival of renal stem cells after birth, probably due to their preference for an hypoxic state, that is abruptly interrupted after delivery[32]. Stimulating endogenous renal stem cells to produce new nephrons might represent a new relevant tool, able to improve the nephron number in preterm and in low birth weight newborns, allowing them to escape their susceptibility to undergo renal insufficiency later in life.
In conclusion, in our opinion, changes are expected both for nephrologists and for neonatologists, regarding regenerative medicine and prevention of kidney diseases.Nephrologists should be more interested to clinical data regarding the first months of life in the womb and in postnatal life of their adult patients, being particularly focused on birth weight and on the weeks of gestation at birth, without forgetting data regarding maternal status during gestation and perinatal asphyxia.Any preterm or low-birthweight women becoming pregnant should be monitored closely for gestational weight gain, fetal growth, and pre-eclampsia.
Neonatologists should be aware that any preterm or low birth weight infant should be considered as a subject with fewer glomeruli, probably predicted to develop renal disease later in life. Similarly, intrauterine growth restriction, or birth after pre-eclampsia or gestational diabetes should be recorded as risk factors for later-life hypertension and chronic kidney disease.
Thus, by a practical point of view, a baseline renal ultrasound, andfollow-up should be performed; regular monitoring of preterm and low-birthweight individuals throughout life is recommended. Exclusive breastfeeding should be promoted in the first 6 months, and other foodsources should be introduced prudently (reduced sodium, carbohydrates, and saturated fat) to allow regular and balanced growth.Annual blood pressure measurement are suggested together with urinalysis[33, 34].Rapid catch-up growth should be avoided to prevent obesity-associated exacerbation of renal risk.Moreover, avoidance of nephrotoxins is important.
Finally, it has been observed in some animal models that low nephronnumbers may also occur with normal birth weight, so the burden or risk of renal programming may be underestimated if birth weight is the only surrogate marker considered [35].The challenge for both neonatologists and nephrologists is to develop an integrative approach, aimed at favouring an ongoing nephrogenesis after birth, at maintaining the self-renewal potential of renal stem cells allowing them to originate new nephrons till the 36th post conceptional week, that represents the physiological stop for human nephrogenesis.
Recently the presence of stem cells (hBSCs: human breastmilk-derived stem cells) and epithelial progenitors has been demonstrated in mother’s milk (MM). Stem cells present in samples of fresh MM exhibit a high degree of vitality. The enormous potential of the use of MM stem cells is the presence of a cell population capable of differentiating into the three mesoderm, endoderm and ectoderm lines. This could be of great potential interest for renal physiologic regenerative medicine from an exogenous source [36-39].
The goal of our preventive regenerative renal approach, to be activated at birth, is to allow preterm and low birth weight infants toreach a sufficient number of functioning nephrons, protecting their nephrogenesis till the physiological end.
With our approach, oligonephronic infants, susceptible to develop ESRF in adulthood, could potentially be transformed into normonephronic subjects, resistant to develop renal disease.
Conflicts of Interest
Authors declare no conflicts of interest.
References
Gerosa C,Fanni D, Nemolato S, et al. Molecular Regulation of Kidney Development in Kidney Development in Renal Pathology, ed: Springer, 2014; 13-28.
Faa G, Gerosa C,Fanni D, et al.Marked interindividual variability in renal maturation of preterm infants: lessons from autopsy.J MaternFetal Neonatal Med. 2010; 23: 129-133.
Faa G,Fanni D, Gerosa C, et al. The subcapsular blue strip width: a new marker for evaluating the residual potential nephrogenesisin the newborn kidney. Laboratory invest. 2013; 93: 387A-387A.
Faa G, Gerosa C,Fanni D, et al. Morphogenesis and molecular mechanisms involved in human kidney development. J Cell Physiol. 2012; 227: 1257-68.
Faa G, Gerosa C,Fanni D, et al. Development of the Human Kidney: Immunohistochemical Findings,” in Kidney Development in Renal Pathology, ed: Springer. 2014; 29-41.
Faa G, Fanos V, Floris G, et al. Development of the human kidney: morphological events,” in Kidney development in renal pathology, ed: Springer, 2014; 1-12.
Fanni D, Gerosa C, Vinci L, et al. Interstitial stromal progenitors during kidney development: here, there and everywhere. J MaternFetal Neonatal Med. 2016; 29: 3815-20.
Fanni D,Sanna A, Gerosa C, et al. Each niche has an actor: multiple stem cell niches in the preterm kidney. Ital J Pediatr. 2015; 41: 78.
Rodriguez MM, Gomez AH, Abitbol CL, et al. Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. PediatrDevPathol. 2004; 7: 17-25.
Puddu M, Fanos V, Podda F, et al. The kidney from prenatal to adult life: perinatal programming and reduction of number of nephrons during development. Am J Nephrol. 2009; 30: 162-70.
Ingelfinger JR. Disparities in renal endowment: causes and consequences.AdvChronic Kidney Dis.2008; 15: 107-14.
Fanos V, Puddu M, Reali A,et al. Perinatal nutrient restriction reduces nephron endowment increasing renal morbidity in adulthood: a review. Early Hum Dev. 2010; 86: 37-42.
Merlet-Benichou C, Gilbert T, Vilar J, et al. Nephron number: variability is the rule. Causes and consequences. Lab Invest. 1999; 79: 515-27.
Georgas K, Rumballe B, Valerius MT, et al. Analysis of early nephron patterning reveals a role for distal RV proliferation in fusion to the ureteric tip via a cap mesenchyme-derived connecting segment.Dev Biol. 2009; 332: 273-86.
Bertram JF, Douglas-Denton RN, Diouf B, et al. Human nephron number: implications for health and disease. PediatrNephrol. 2011; 26: 1529-33.
Luyckx VA, Brenner BM. Low birth weight, nephron number, and kidney disease. Kidney Int Suppl. 2005; S68-77.
LuyckxVA, Brenner BM. The clinical importance of nephron mass. J Am SocNephrol. 2010; 21: 898-910.
Puddu M, Cataldi L, Faa G, et al. Perinatal programmino: long-term consequences for kidney. 2011.
Moritz KM, Wintour EM, Black MJ, et al. Factors influencing mammalian kidney development: implications for health in adult life.AdvAnatEmbryol Cell Biol. 2008; 196: 1-78.
Fanni D, Gerosa C, Nemolato S, et al.Physiological renal regenerating medicine in VLBW preterm infants: could a dream come true?.J MaternFetal Neonatal Med. 2012; 25: 41-48.
Faa G, Gerosa C, Fanni D, et al. The role of immunohistochemistry in the study of the newborn kidney. J MaternFetal Neonatal Med.2012; 25 :135-8.
Fanni D, Fanos V, Monga G, et al. MUC1 in mesenchymal-to-epithelial transition during human nephrogenesis: changing the fate of renal progenitor/stem cells?.J MaternFetal Neonatal Med. 2011; 24: 63-66.
Fanos V, Castagnola M, Faa G. Prolonging nephrogenesis in preterm infants: a new approach for prevention of kidney disease in adulthood?. Iran J Kidney Dis. 2015; 9: 180-5.
Benigni A, Morigi M, Remuzzi G. Kidney regeneration. Lancet. 2010; 375:1310-7.
Chou YH, Pan SY, Yang CH, et al. Stem cells and kidney regeneration. J Formosed Assoc. 2014; 113: 201-9.
Remuzzi A, Figliuzzi M, Bonandrini B, et al. Experimental Evaluation of Kidney Regeneration by Organ Scaffold Recellularization. Sci Rep. 7: 43502.
Chevalier RL, Charlton JR. The human kidney at birth: structure and function in transition,” in Kidney development in renal pathology, ed: Springer. 2014; 49-58.
Hughson M, Farris AB, Douglas-Denton 3rd R. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int.2003; 63: 2113-22.
Bertin M, Fanos V, Zanardo V. Malnutrition and Renal Function, in Kidney Development in Renal Pathology, ed: Springer. 2014; 95-102.
Luyckx VA. Preterm Birth and its Impact on Renal Health.SeminNephrol. 2017;37: 311-319.
FaaG, Sanna A, Gerosa C, et al. Renal physiological regenerative medicine to prevent chronic renal failure: should we start at birth? ClinChimActa. 2015; 444: 156-62.
Gerosa C, Fanni D, Faa A, et al. Low vascularization of the nephrogenic zone of the fetal kidney suggests major role for hypoxia in human nephrogenesis. IntUrolNephrol. 2017; 49: 1621-1625.
Luyckx VA, Perico N, Somaschini M, et al. A developmental approach to the prevention of hypertension and kidney disease: a report from the Low Birth Weight and Nephron Number Working Group. Lancet.2017; 390: 424-428.
The Impact of Kidney Development on the Life Course: A Consensus Document for Action. Nephron.2017; 136:3-49.
Gilbert JS, Lang AL, Grant AR, et al. Maternal nutrient restriction in sheep: hypertension and decreased nephron number in offspring at 9 months of age. J Physiol. 2005; 565: 137-147.
Fanos V. Metabolomics, milk-oriented microbiota (MOM) and multipotent stem cells: the future of research on breast milk.J PediatrNeonat Individual Med. 2015; 4: e040115.
Pichiri G, Lanzano D, Piras M, et al. Human breast milk stem cells: a new challenge for perinatologists.J PediatrNeonat Individual Med. 2016; 5: e050120.
Fanos V, Loddo C, Puddu M, et al.From ureteric bud to the first glomeruli: genes, mediators, kidney alterations. IntUrolNephrol. 2015; 47: 109-16.
Reali A, Puddu M, Pintus MC, et al. Multipotent stem cells of mother’s milk. J PediatrNeonat Individual Med.2016; 5: e050103.
Received: June 24, 2018;
Accepted: July 10, 2018;
Published: July 13 , 2018
To cite this article : Faa G, Fanni D, Gerosa C, et al. Kidney development and susceptibility to develop kidney disease in adulthood. Japan Journal of Medicine. 2018: 1:4.
© Faa G, et al. 2018.
