Review Article / Open Access
DOI: 10.31488/jjm.1000108
Neonatal Menstruation and its Meaning
Paola Bianchi1 , Ivo Brosens2 , Giuseppe Benagiano3
Department of Medico-Surgical Sciences and Traslational Medicine, Sapienza, University of Rome, Sant’Andrea Hospital, Rome, Italy.
Department of Obstetrics and Gynecology, Catholic University of Leuven, Leuven, Belgium.
Department of Obstetrics, Gynecology and Urology, Sapienza, University of Rome, Rome, Italy.
*Corresponding author:Dr. Paola Bianchi, Via di Grottarossa 1035, 00135, Roma, Italy.
Abstract
Neonatal uterine bleeding (NUB), or neonatal menstruation is today a totally neglected phenomenon, labelled as “perfectly normal and therefore not worth investigating”. Yet, over the last two centuries, its frequency, characteristics and pathophysiology have been carefully investigated, leading to the conclusion that its occurrence is a strong marker of fetal distress.Cohort studies have found that its frequency among healthy, term newborns is around 5%. However, when the presence of minute quantities of blood was investigated, this was found in as much as two-thirds of neonates.The frequency of NUB is lower in infants born prematurely, but is higher in low birth-weight babies, in those born after a gestation complicated by preeclampsia or by feto-maternal incompatibility and in those born post-term.The reason for the low percentage of newborns experiencing NUB lies in a lack of response of the fetal endometrium, even late in pregnancy, to the presence of ever increasing levels of circulating progesterone, a phenomenon known as “ontogenetic progesterone resistance”. In fact, histologically, a full response with decidual transformation can be observed in only some 5% of neonates (the same frequency of overt NUB). When NUB occurs, because of the peculiarities of the long fetal cervical canal, it is likely that some menstrual blood will reflux in to the peritoneal cavity where, if endometrial stem/progenitor cells are present, they can lie dormant in a niche, becoming active at thelarche and, in specific instances, give rise to an early-onset variant of endometriosis (EOE).In view of this possibility, two actions should be taken by the scientific community: institute universal recording of NUB in neonatal units; and carry-out large, controlled trials to verify the theory of a neonatal origin of EOE.
Keywords: neonatal menstruation; neonatal uterine bleeding; progesterone resistance; defective decidualization
Introduction
The phenomenon of “neonatal menstruation” has been described in the English literature as “neonatal uterine bleeding” (NUB), by French authors as “la crise génitale du nouveau-né” and in German as “Neugeborenen genitalkrisen”.
Scientific interest for this unusual type of bleeding was high during the XIX and first half of XX century and faded away thereafter. Such a lack of interest clearly stems from the widespread belief,expressed in the lay press, that NUB is the obvious consequence of the sudden drop in neonatal circulating steroid hormone levels that follows birth and the consequent detachment of the placenta. At the same time, in spite of a lack of modern original studies on NUB, a lively discussion on this topic can be found on the internet. For instance, the WebMD site explains clearly: “Your newborn girl’s genitals have been exposed to many hormones in the uterus. Among other things, these hormones may have made the outside of the vagina (“labia majora” and the “clitoris”) a little swollen and prominent and caused a thick, milky discharge in the vagina. Most dramatically, at 2 or 3 days of age, your daughter may have a little bit of bleeding from her vagina. This is perfectly normal – it is caused by the withdrawal of the hormones she was exposed to in the womb. It will be her first and last menstrual period for another decade or so”[1].
Clearly, the scientific community seems today to agree that NUB deserves no attention, given the total absence of scientific publications dealing with the subject since 1985, when a Yugoslav team reported the results of a study of all neonates born in the city of Novi Sad in 1979 [2]. One negative consequence of this neglect is that NUB is not recorded in clinical birth notes.
This attitude, however, completely ignores a simple fact: the sudden, major drop in circulating progesterone (P) levels that follows placental detachment occurs in all female newborns, whether preterm, at term of post-mature. Yet, it is common knowledge that NUB is a relatively rare phenomenon, occurring in not over 5% of all female neonates [3, 4]. This rare occurrence should have driven attention to the fact that other factors must be involved and should have discounted the simplistic view considering it a “perfectly natural phenomenon”.Intriguingly, the answer to the question had been available for decades in a large body of data published over almost 200 years. Indeed, a careful search of the literature shows that at least 80 publications, including case reports, small and large series, cohort investigations and pathology findings were published between 1822 and 1985.As it should be obvious, this old literature is not found in any electronic data-base and must be found through a manual search.
At any rate, published information details not only the characteristics of NUB and its frequency in various fetal conditions, but also the pathogenetic mechanisms leading to the shedding of neonatal endometrium.Reconstructing the path of the discovery of NUB and of the circumstances surrounding it, is not a purely academic exercise, since clear links have now been found between NUB and several conditions manifesting themselves later in life [5-8]. Unfortunately, in the absence of any recording, prospective studies to prove or disprove a relationship between NUB and events later in life cannot be carried out and, even if recording is started, it will take many decennia before results from epidemiological studies will become available [9].
Here we wish to reconstruct both the discovery and the evaluation of the occurrence of NUB, and also the possible consequences that its occurrence may have on the adolescent and adult woman who had experienced it at birth.
Historical overview
The careful search of old publications identified a review of the early literature by Cullingworth[10] who, in 1876, provided in a table full information on the subject. He reported that the first mention of NUB in an eight days old infant, is contained in a Book by Louise Bourgeois, published in France in 1642 [11]. This was followed by a description by Weisius in Germany in 1650 [12]. Cullingworth lists 12 references before the year 1800; they seem an array of various forms of bleeding, including one case published by Bohnius in 1686 in Leipzig [13] in which the bleeding appeared monthly until age 12.
The first mention of NUB in a scientific journal was made in a brief note published by Carusin 1822 [14]; he reported on a case observed in March 1819. Possibly what drew the attention of this German obstetrician was the fact that the woman four days after delivering her third child suffered from a strong vaginal bleeding, “which stopped after drinking several cups of cinnamon tea”. Then, 6 days after delivery, Carus observed the presence of a vaginal mucus discharge in the female neonate, followed the next day by “drops of blood” that continued for three days. During this period,the bleeding “was quite similar to a menstruation” and the baby looked perfectly healthy.
Several additional articles, dissertations and books were published during the second part of the XIX century. Scientific investigations on NUB intensified during the XX century. Of particular importance is the work of Halban[15]who, in 1904, not only described in detail the phenomenon, but provided an explanation for what he considered a “physiological event” due to a reaction to active substances produced during pregnancy by the placenta. He further speculated that these substances are then secreted to both the mother and the fetus, where they induce changes in the target organs. He believed that the fetal uterus reacts with decidualization in a way similar to that of the maternal uterus; however, the fetal endometrium reacts in a weaker way and therefore the menstrual-like changes are milder and occasional. After birth, following the separation of the placenta from both the mother and the fetus, these substances no longer reach either organism causing the well-known puerperal involution. This, in the neonatal organism may take the form of a menstruation and therefore the phenomenon deserves to be identified as ‘neonatal menstruation’ given the presence of a similar endometrial response (decidualization) as in adult menstruation.
During the first part of the XX century a number of reports were published in England [16, 17], Germany [18, 19], Italy[20, 21] and in France [22, 23] where neonatal menstruation became part of a syndrome identified as “the newborn’s genital crisis” that involved other organs as well, such as the breast.
The careful search of old publications identified a review of the early literature by Cullingworth[10] who, in 1876, provided in a table full information on the subject. He reported that the first mention of NUB in an eight days old infant, is contained in a Book by Louise Bourgeois, published in France in 1642 [11]. This was followed by a description by Weisius in Germany in 1650 [12]. Cullingworth lists 12 references before the year 1800; they seem an array of various forms of bleeding, including one case published by Bohnius in 1686 in Leipzig [13] in which the bleeding appeared monthly until age 12.
The first mention of NUB in a scientific journal was made in a brief note published by Carusin 1822 [14]; he reported on a case observed in March 1819. Possibly what drew the attention of this German obstetrician was the fact that the woman four days after delivering her third child suffered from a strong vaginal bleeding, “which stopped after drinking several cups of cinnamon tea”. Then, 6 days after delivery, Carus observed the presence of a vaginal mucus discharge in the female neonate, followed the next day by “drops of blood” that continued for three days. During this period,the bleeding “was quite similar to a menstruation” and the baby looked perfectly healthy.
Several additional articles, dissertations and books were published during the second part of the XIX century. Scientific investigations on NUB intensified during the XX century. Of particular importance is the work of Halban[15]who, in 1904, not only described in detail the phenomenon, but provided an explanation for what he considered a “physiological event” due to a reaction to active substances produced during pregnancy by the placenta. He further speculated that these substances are then secreted to both the mother and the fetus, where they induce changes in the target organs. He believed that the fetal uterus reacts with decidualization in a way similar to that of the maternal uterus; however, the fetal endometrium reacts in a weaker way and therefore the menstrual-like changes are milder and occasional. After birth, following the separation of the placenta from both the mother and the fetus, these substances no longer reach either organism causing the well-known puerperal involution. This, in the neonatal organism may take the form of a menstruation and therefore the phenomenon deserves to be identified as ‘neonatal menstruation’ given the presence of a similar endometrial response (decidualization) as in adult menstruation.
During the first part of the XX century a number of reports were published in England [16, 17], Germany [18, 19], Italy[20, 21] and in France [22, 23] where neonatal menstruation became part of a syndrome identified as “the newborn’s genital crisis” that involved other organs as well, such as the breast.
Cohort studies
The results of several cohort studies have been published between 1964 and 1985. After this, no additional information on neonatal menstruation seems to have been published.
Newborns from healthy pregnancies
The first systematic study of neonatal menstruation was presented to the French Pediatric Society in 1963 by Lévy et al. [24]. This group evaluated NUB’s frequency in a population of 1’057 healthy female babies born at the Strasbourg Maternity Hospital between February 1962 and 1963 and observed visible bleeding in 48 of them (4.5%).In addition, there were 150 babies necessitating neonatal medical care. Among them 9 (6%) presented with NUB. Their characteristics were as follows: 86 (2, or 2.3% with NUB) were premature and 64 were at term (7, or 10.9% with NUB). Overall, in the newborns from physiological pregnancies NUB occurred in 4,72 % of the total.
Interestingly, the incidence of neonatal menstruation in the low birthweight group was 6.2%, higher than the control group, although not significantly so (p = 0.22). Unfortunately, gestation length was not recorded in this study, rendering it impossible to separate premature from small-for-gestational-age newborns. A major handicap, since there is evidence from other studies that a healthy neonate born prematurely would behave differently from a low-for-gestational-age baby born around term.
In 1968, Zubovich [25] reported that in a population of 541 newborn females NUB was macroscopically visible in 7.8% and microscopically in as much as 66%.Subsequently, Kaiser et al. [3] evaluated 153 neonates and found very similar results: NUB was visible in 5.3% and – through detection of hemoglobin – it was microscopically present in 61.3% of the cases. In terms of its major features, bleeding usually started 3 to 7 days following delivery and on average lasted 3.2 days.
Additional information became available in 1976 when Huber [4] examined 350 newborn girlsand visually observed the presence of bleeding in 3.3% of the cases. However, when he repeatedly looked for occult bleeding during the first week post-partum, its presence could bedetected 719 times using the test strips utilized to check for blood in the urine.Overall, a positive reaction was found in 25.4% of the neonates. Furthermore, erythrocytes were observed in only two cytological preparations on days 6 and 7. Huber warned that using these ‘strips’ may cause surface injuries to the delicate vaginal epithelium and that for this reason, the test should be made only at the vaginal vestibule. They evaluated the timing of NUB and recorded when the first, even slight discoloration of the test strip occurred (Figure1). The highest frequency of bleeding is reached on the 5th day post-partum.
The largest study ever carried out, as well as the last published, is the work of pediatricians at the University of Novi Sad in Serbia [2]. During 1979, they carefully observed a total of 2477 newborn female infants of whom 2241 (90.47%) were born at-term. Visible NUB could be observed in 3.79% of them.In terms of timing, the smallest percentage of bleeding occurred on day 1 and 7 postnatally (3.89%), whereas bleeding was most frequent on day 5 (40.26%) and on day 4 (18.18%) postnatally.Data for healthy infants are summarized in table 1.
Newborns from complicated pregnancies
In 1962, the Strasbourg Group published an investigation dealing with the characteristic of neonates from pre-eclamptic mothers and noted the great frequency of NUB in these newborn girls. Indeed, among 117 neonates at term from toxemic mothers, they observed 22 cases (18.8%) of NUB[26]. This prompted them to investigate factors capable of modifying the frequency of neonatal bleeding [24].They studied a total of 272 female babies from complicated gestations, observing NUB in 38 of them (13.9%). They focused their attention over three factors that seemed to increase the frequency of NUB: Feto-maternal incompatibility (Rh, AB0) where 7 out of 49 (14.3%) had NUB; toxaemia, where 19 out of 40 (47.5%)had NUB; post-maturity, where 7 out of 13 (53.8) had NUB. In contradistinction to this, they studied 584 premature babies among whom NUB occurred in 36 (6.16%).Apparently, this group comprised both pre-term and SGA cases, thereby confusing the issue.
The same variables were also evaluated in the cohort investigated by Berić et al. [2], where results were partially at variance with those of Lévy et al. [24], in that they found that NUB was rarely found in premature (pre-term) new-borns (0.79%), was more common in term babies (3.79%) and most common in post-term new-borns (9.09%). In preterm neonates bleeding usually occurred later (on the 5th day) and in post-term infants it lasted longer.
Mechanism of neonatal bleeding
In a volume dedicated to the reproductive endocrinology of the human fetus and published in 1955 in France, Rosa [27] described the development of the endometrium in a series of 31 fetuses aged between 4½ months and term. He found that during the 5thmonth of fetal life the endometrium is morphologically well formed, but both the stroma and the glands seem to be inactive. As pregnancy proceeds – although estrogens circulate in the fetus mostly in a conjugated, inactive form [28, 29] – the endometrium begins to react to the estrogenic stimulus and a response can be observed between 5 and 7½ months.By the 8th month,the increasing circulating levels of progesterone [30] also begin to produce some effect on the endometrial glandular compartment; this takes the form of coiled glands, apocrine secretions and glycogen enclaves. It seems therefore that, although during the second half of pregnancy the fetal endometrium begins to respond in a way similar to what happens during the adult menstrual cycle, significant differences also exist: glands are poorly developed and the stroma does not show decidual changes in spite of the huge amounts present [27].
Table 1. Prevalence of visible and occult NUB.
| ClinicalPresentation | Incidence (%) | References |
|---|---|---|
| Visible | 4.77.8
5.3 3.3
|
[24][25]
[3] [31]
|
| Occult
|
66.0 61.3 25.4 |
[25][3]
[4] |
Further information was later provided by Huber et al. [31] who examined 82 uteri from fetuses, infants and children and documented that, prior to the 20th gestational week, no glandular development takes place. They confirmed that during the second half of gestation cellular differentiation of glands and stroma gradually begins.
The full picture of the endometrium in female fetuses around parturition has been provided by Oberet al. [32] in a comprehensive study of 169 female newborns. Their observations show that, in spite of the very high levels of progesterone circulating in the fetus around term, in a majority of cases (65%), the endometrium failed to properly respond. Indeed, secretory activity was found in only 27% of the neonates, with full decidual changes present in some 5% of them. Finally, in a mere 3% of the cases there were typical menstrual changes with clotted blood in the endometrial cavity; interestingly, all these babies had died within 3 days of birth, indirectly indicating that their pregnancy was far from being physiological. The two pathologists concluded that only in a few instances the fetus could fully react to placental progesterone producing secretory and decidual transformations in the endometrium. Another investigation covering changes occurring towards the end of pregnancy and in the neonate, is that by Huber et al. [31]. They confirmed that, from the 34thweek onward, secretory endometrial changes can be found. In some fetuses these changes progressed until birth, with the formation of tall cylindrical epithelial cells with clear cytoplasm and visible glycogen deposits, as well as the presence of mucin in the lumen.
In another study, Pryse-Davies et al.[33] examined 145 newborns and in the babies at term found menstruation-like changes in the uterine fundus with hemorrhage into the uterine cavity in only 2 of the 55 neonates examined.
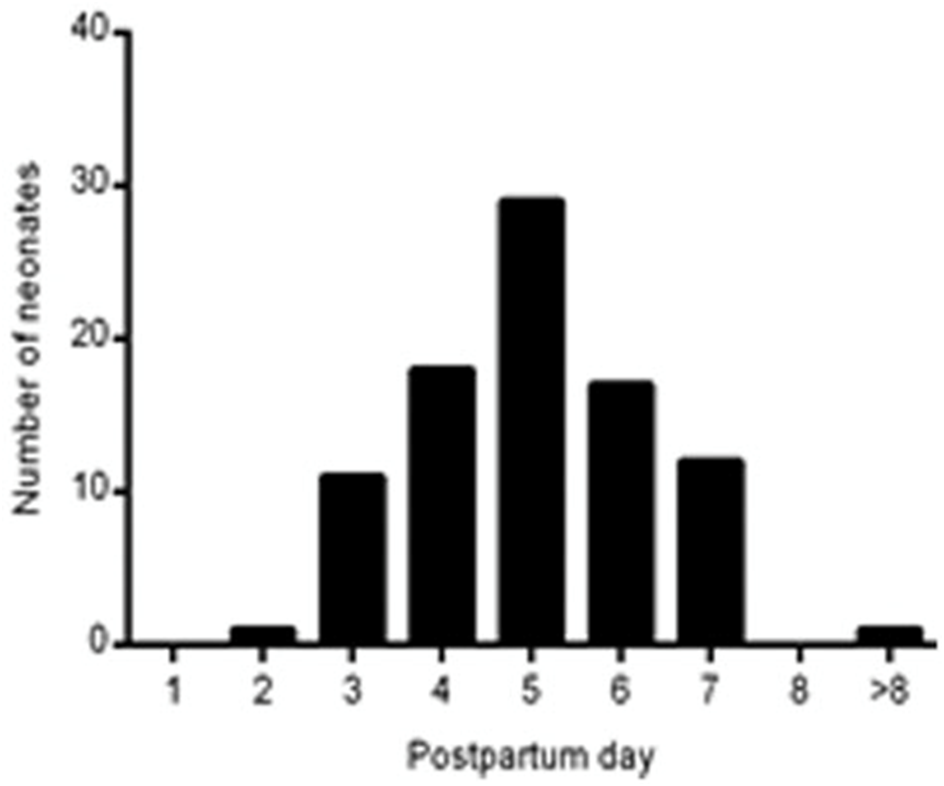
Figure 1.Distribution of the day of appearance of neonatal bleedingaccording to Huber [4].
Finally, it has been documented that during the second half of fetal life, nuclei of endometrial glands in the corpus uteri increase in size, plateauing at term and immediately after delivery [34]; also, the nuclei of endometrial stromal cells grow during fetal life, reaching a maximum towards the end of pregnancy [35].These studieshave provided a logical explanation for the rarity of neonatal menstruation, in spite of the sudden, substantial drop in circulating P in all newborns: somehow, the endometrium of the vast majority of normal neonates in incapable to respond to the action of progesterone. This phenomenon, however, was identified and studied in detail only years later.
In his investigation, Huber reported also on the regression of the endometrium after birth, showing that by the second week post-partum, no glandular activity is present and glycogen has disappeared [31].Additional information has been provided by Kaiser and Grässel[3] and Kaiser et al. [36] who showed that within 5 days of delivery there is disintegration of the glandular structure and that throughout infancy and early childhood, the endometrium consists of a thin, atrophic, epithelial layer and scant stroma. It is not until the 8th year of life that the situation of complete inactivity begins to change, showing an increased variability in nuclear size between subjects; this in turn seems to reflect a cellular responsiveness to a weak hormonal stimulus. Finally, following delivery the nuclei of endometrial cells and stroma reduce in size and until the 7th year of life, no change was observed [34, 35]. The modifications occurring in the uterus from birth to menarche are summarized in figure 2.
It is important to stress that in contradistinction to the uterus, ovaries remain inactive during fetal life. This observation was first made by Spivak in 1934 [37] who, after observing that in neonates, ovaries present large cystic structures, concluded that they were nonetheless inactive and did not play any role in determining the condition of the endometrium. This conclusion was fully shared by Oberet al. in their detailed study of fetal ovaries [32]. They observed only one case of follicular development to the antral stage and no Graafian follicles or corpora lutea. In the already-mentioned study,Pryse-Davies et al. [33] based on ovarian and endometrial specimens from 145 fetuses and neonates (36 to 42 weeks old), reached similar conclusions. However, in contradistinction to the findings of Ober et al. in addition to cystic structures they observed also Graafian follicles. Luteinization of the theca (attributed to an increased production of hCG) was occasionally observed. In their view, although there was some correlation between Graafian follicle formation and endometrial vacuolization, this may have been due to the fact that the frequency of both increases with gestationalage and concluded that there was no direct correlation between ovarian changes and endometrial secretory changes.
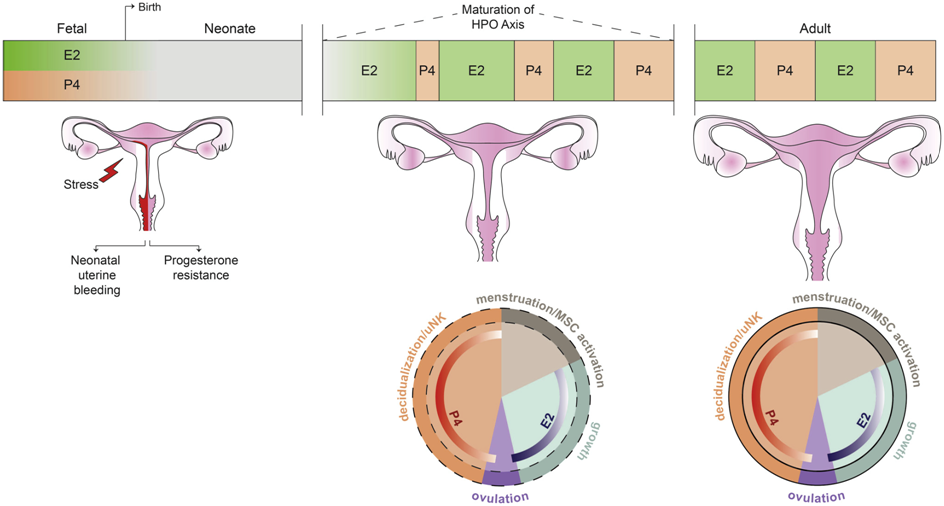
Figure 2.Uterine maturation from birth till adulthood [66].
Progesterone resistance and its genesis
The knowledge of the existence of resistance to the action of progesterone dates back 40 years when the case was presented of a young infertile woman who, in repeated late luteal phase endometrial biopsies, showed glandular stromal dissociation with failure to undergo decidualization, in spite of a normal P serum profile. Intriguingly, this abnormality could not be corrected by P administration and, compared to two normal controls, a careful biochemical evaluation of the endometrium of the subject evidenced that the number of high affinity P-binding sites in the cytosol fraction was some 50% lower [38]. At the time it was felt that this phenomenon might have been due either to an absence or a reduction in the number of stromal cytosol receptors, or to a resistance to the specific hormone action, or both.
The concept of ‘progesterone resistance’ was then defined in 1986 [39] and it implies a decreased responsiveness of target tissues to bioavailable P; this phenomenon can be observed in cancer patients [40-42], in women with adenomyosis [43], endometriosis [44], PCOS [45] and, as mentioned, in a majority of neonates [32]. In this last case, the expression “ontogenetic progesterone resistance” has been coined [46] to signify that the phenomenon is inscribed in the developmental history of the fetus. As knowledge of the condition improved, it has been argued that the expression is a misnomer, since the phenomenon is associated with a modification of a series of key endometrial signal transduction pathways [47].Indeed, when these abnormalities are used as a marker, the phenomenon also occurs in women with recurrent pregnancy loss [48]. This may signify that steroid hormone responses by the endometrium are likely to be much more dynamic and complex than previously appreciated. Progesterone resistance, as manifested in conditions like endometriosis, is not just a consequence of perturbed progesterone signal transduction caused by chronic inflammation but is associated with long-lasting epigenetic reprogramming of steroid hormone responses in the endometrium and beyond.
It has been suggested that estrogens and their receptors (ER) may be involved in uterine maturation: Glatsteinet al. [49] have investigated the pattern of ER gene and protein expression in the human fetal uterus and found that is expressed within the uterine mesenchyme at the interface of the differentiating endometrial stroma and myometrium. This highly specific location of the ER protein, together with the messenger ribonucleic acid data suggests a role in the differentiation of the primitive uterine mesenchyme into stromal and myometrial compartments. Unfortunately, there seem to be no studies on progesterone receptor (PR) expression in the human fetal uterus, althoughCalhoun et al. [50] reported that PR expression was extremely poor in the fetal rhesus macaque endometrium a little past midgestation, increasing significantly at term, but only to 15%.
In a recent opinion [8], we have suggestedpossible models to explain theincreased P responsivenessat term, or soon after birth. A first option is that,through some as yet unknown mechanism,PR expression is upregulated, eventually leading to a decidual response. Another possibility is that the responsivenessof the endometrium is connected tothe switching of theproduction of hematopoietic stem cellsfrom the fetal liver to the bone marrow that occurs late in pregnancy. Therefore, it is notinconceivable that the establishment ofa functional stem cell niche environmentin bone marrow bestows thenecessary competence on mesenchymal stem cells tomigrate to the uterus, populate thespace around the immature spiral arteries,and induce a progesterone responsive microenvironment.
Several cell populations seem to play a role in the progressive maturation of the response to P by fetal and neonatal endometrium. During the second part of a gestation, the most abundant endometrial leukocyte populations are CD14þ monocytes and CD68þ macrophages [51].This is contrasted by the absence of CD56þ uterine natural killer (uNK) cells that, as shown by clear evidence, are an important source of angiogenic and other factors necessary for the physiological transformation of spiral arteries at the maternal-fetal interface [52]. At birth, however, also uNK cells appear in the endometrium, although in a small quantity [53].
These observations lead to the conclusion that the stromal compartment of the human endometrium becomes gradually responsive to signaling by P around the very end of gestation. This conclusion seems reinforced by the already mentioned higher frequency of NUB in post-term babies [2,24].
In conclusion, we believe that the human endometrium must transition from a stage of fetal progesterone resistance to full progesterone responsiveness in the adult uterus. When and how exactly this happen remains speculative.
Peculiarities of neonatal menstruation
Vaginal menstrual-like bleeding may represent only part of the phenomenon of NUB. This is because the anatomy of the uterus towards the end of pregnancy is very different from that of the adult organ. Decades ago, German investigators have provided important details on this subject:At the beginning of the second trimester of pregnancy the fetal urethra, vagina, uterus and Fallopian tubes begin to take shape and have a defined lumen [54]. Then, during the third trimester there is atremendous growth of the uterine cervix and of the vagina, untilat birth the length of the vagina is estimated to be 4 cm and that of the cervix between 2 and 2.5 times the corpus uteri that, apparently, does not grow during the last part of gestation [55, 56].
Another intriguing phenomenon occurs after the 26thweek of gestation: the cervical canal is no longer patent, presumablybecause of the presence of a plug due to secretions by the cervical epithelium lining the canal [54]. It has been speculated that this functional plug may facilitate a retrograde reflux of the uterine bleeding, when present, into the newborn peritoneal cavity. Also, the onset of visible bleeding may become unpredictable if not obscured. Another important feature is that a sort of filtration process through the long cervical canal may produce a predominantly acellular fluid that, because of the lysis of red blood cells, may be stained with hemoglobin [3].
To be clear, there is no proof that such a reflux indeed occurs; however, the presence of a ‘cyclical blood staining of peritoneal dialysis fluid’ in pre-menarcheal girls undergoing peritoneal dialisis ‘prior to any vaginal bleeding’ [57], makes plausible the hypothesis that in the neonate vaginal bleeding is preceded by retrograde bleeding.The hypothesis is summarized in Figure 3.
The importance of this phenomenon becomes evident when considering that endometrial stem/progenitor cells have been identified in the menstrual blood of adult women [58], suggesting that they may also be shed during NUB, seeding the peritoneal cavity, lying dormant until menarche and becoming active soon after [7].
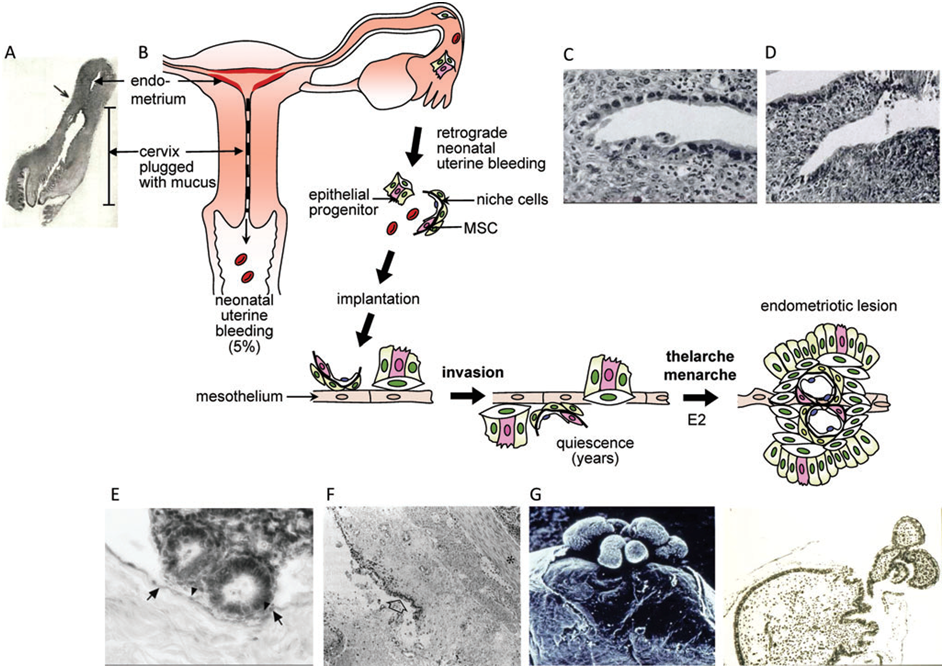
Figure 3.Schematic description of the hypothesis that endometrial stem/progenitor cells may play a role in early-onset endometriosis with supporting imagesfrompublishedworks. (A)Neonatal uterus andvaginashowing relatively long cervix incomparisonto the uterine body. The arrowindicates the corpus-cervicaljunction. Mucus has been removed from the cervix. (B) Schematic image showingneonatal uterine bleeding (which occurs in 5% of neonates) and the hypothesizedretrograde neonatal bleeding due to cervical obstruction by thick mucus in the long neonatal cervix. The fragments of shed endometrial tissue are postulatedto contain an endometrial epithelial progenitor cell (pink) and a perivascular mesenchymal stem/stromal cell (MSC) (pink) together with niche cells. Theserapidly adhere to the neonatal mesothelium, invade and/or become contiguous with the mesothelial lining where they remain in a quiescent state for some 10years. Rising estrogen (E2) levels associated with thelarche and menarche reactivate the stem/progenitor cells to initiate the growth of endometrioticlesions on the surface of or below the peritoneal mesothelium. Neonatal (C) decidualized and (D) shedding endometrium. (E) Endometrial attachment tothe mesothelium can occur within 1 h and (F) implantation by 18 h, with endometrial cells becoming contiguous with the mesothelium (arrow) before theonset of quiescence. (G) Scanning electron microscopy (left) and histological section (right) of a peritoneal endometriotic implant showing a polypoidlesion extending through the mesothelium in a young girl after a decade of quiescence.
Images reprinted with permission from: Lippincott Williams andWilkins/Wolters Kluwer Health, (A) Figure 3 from Fluhmann[55]and (G) Figures 1 and 2c from Cornillie et al. (1986); American Academy of Pediatrics,
Lack of decidualization and its consequences
It is common knowledge that withdrawal of progesterone action from a decidualizing endometrium is the specific signal starting the cascade of events that produce menstruation [59]. Within this context, probably the most striking feature of the fetal endometrium is the lack of decidual transformation of the stroma despite prolonged exposure to estrogens and progesterone.
Decidualization is a process driven by P and characterized by the transformation of endometrial stromal fibroblasts into specialized secretory decidual cells. It is a modification considered indispensable for the establishment of a viable pregnancy, because decidual cells provide both a nutritive and immuno-privileged matrix essential for embryo implantation and placental development.In the absence of a neonatal menstruation, it seems likely that the endometrium remains partially immature with a first wave of endometrial shedding, followed by regeneration taking place only after the onset of menarche. In other words, it has been hypothesized that the human uterus per se is initially an immature organ that acquires competence in response to dynamic remodelling events triggered by NUB, anovulatory bleeding, menstruation, miscarriage or parturition [60].
This situation poses intriguing questions, since it has been hypothesized that cyclicwaves of endometrial regeneration may be required for the establishment ofa fullresponse to ovarian hormones. This postulates that tissue shedding and menstrual bleeding act as ‘preconditioning events’ for a normal pregnancy [61]. For these reasons, we have suggested that suboptimal responsiveness of perivascular niche cells to deciduogeniccues seem likely to lead to poor trophoblast invasion, inadequateremodelling of spiral arteries and defective deepplacentation [61].
Future consequences on neonatal menstruation
In 2013 we put forward for the first time the hypothesis that NUB might increase the risk of early-onset endometriosis (EOE) [5]. The same year, the hypothesis was further elaborated showing that in addition to the above-mentioned considerations, it has been observed that adolescents with cervical outflow obstruction and patent Fallopian tubes,have a significantly increased risk of endometriosis suggesting that in children and young adolescents, endometriosis may originate from retrograde uterine bleeding soon after birth [6]. It was further suggested that stem/progenitor cells present in neonatal menstrual peritoneal refluxmay play a role in the pathogenesis of EOE. These cells can then survive in the pelvis even in the absence of circulating estrogens supported by niche cells also shed during NUB.Then, during thelarche rising estrogen levels will allow their proliferation and establish the ectopic lesions characteristic of endometriosis [7]. We have also provided evidence that EOE attributable to NUB will results in more florid and progressive disease, characterized by highly angiogenic implants, recurrent ectopic bleeding, and endometrioma formation. [62].
In view of data suggestinga higher incidence of NUB in the presence of fetal stress, we have also proposed that feto-maternal risk factors associated with neonatal menstruation might be useful in identifying women at higher risk of endometriosisand recommended to explore early-life events that may help identifying young women at risk of severe, progressive disease [63, 64]. Finally, we drew attention to the need to prove or disprove a link between EOE and NUB; this could be done through two approaches: well-designed large, prospectivestudies and a systematic registration of neonatal menstruation [9, 65].
Conclusions
We hope that this brief overview of the knowledge accumulated over the last two centuries on neonatal menstruation will achieve two main objectives [46]: First, prove that NUB is not ‘a perfectly physiological phenomenon of no clinical relevance’; on the contrary it is an important sign of fetal distress and of accelerated endometrial maturation. Second, that if the occurrence of NUB may signify a lower risk of complications in the event of an early pregnancy in the future adolescent, it may also signify that the girl may be at an increased risk of EOE.
In summary we see today a double challenge: on the one hand, the already stressed need to test the hypothesis of a neonatal origin of EOE through large, prospective studies aimed at ascertaining whether progesterone response of the neonatal endometrium indeed represents a key factor in determining the future reproductive health in the adolescent girl. Second, to drive attention to the fallacy inherent in the concept proposed by the lay press of the ‘naturality of thelittle bleeding from the vagina’and therefore to the need that NUB becarefully recorded as aunique clinical marker of fetal distress.
In this respect, the sooner registration of the presence or absence of neonatal uterine bleedingbecomes widespread, the better it will be, since it takes at least twodecennia to know the results.
Conflict of Interest
The Authors report no conflict of interest.
References
WebMD. Your Newborn Girl’s Genitals and Bleeding. 2018.
Berić BM, Prodanović Z, Mitrović M, et al.Uterine hemorrhage in newborn.Jugoslav Ginekol Perinatol. 1985; 25:89-98.
Kaiser R, Grässel G. Frequenz et al. Incidence and intensity of uterine bleeding in the neonate. Gebursth Frauenheilk. 1974;34:644-8.
Huber A. The frequency of physiologic vaginal bleeding of newborn infants. Zentralbl Gynakol. 1976;98:1017-20.
Brosens I,Benagiano G. Is neonatal uterine bleeding involved in the pathogenesis of endometriosis as a source of stem cells? Fertil Steril. 2013; 100:622-3.
Brosens I, Brosens JJ,Benagiano G. Neonatal uterine bleeding as an antecedent of pelvic endometriosis. Hum Reprod.2013;28:2893-7.
Gargett E,Schwab KE,Puttemans P, et al. Potential role of endometrial stem/progenitor cells in the pathogenesis of early-onset endometriosis. Mol Hum Reprod. 2014;20:591-8.
Brosens I, Benagiano G, Brosens JJ. The potential perinatal origin of placentation disorders in the young primigravida. Am J Obstet Gynecol. 2015; 212:580-5.
Bianchi P, Benagiano G, Brosens I. Promoting awareness of neonatal Menstruation: An important, yet neglected reproductive phenomenon. Gynecol Endocrinol. 2017;33:173-8.
Cullingworth CJ. Haemorrhage from the Genital Organs in the Recently Born Female Child. Liverpool and Manchester Med Surg J. 1876;4:43-58.
Bourgeois L. Various observations on sterility and various diseases of women and neonates. Paris. 1642, volume II, page 23.
Weisius M. As reported by Sennertus D. Opera Omnia. Tomus Tertius, Lugd. 1650;4: 51.
Bohnius J. Circulus Anatomico-Physiologicus. Leipzig 1686; 243.
Carus CG. Blood out of the birth parts of a new-born child. Ztschr Natür Heilk usw. 1822;2:106-7.
Halban J. Pregnancy reactions of fetal organs and their puerperal involution. Ztschr Gebursth Gynäkol. 1904;53: 191-231.
Jardine R. Menstruation in a newborn child. Brit Med J. 1901;1:340.
Frew W. Menstruation in a newly-born female child with convulsions. Brit Med J. 1902;1:1536.
Kouindjy M. Three cases of menstruation in newborns. Arch Méd Enfants. 1900;13ème série:677-9.
Halban H. About fetal menstruation and its meaning. Berliner Klin Wochenschrift. VII.76 (Versammlung Deutscher Naturvorscher und Aertze in Breslau) 1904;48: 1254-6.
Krönig Frl.Metrorrhagia in neonates. Münch medWochensch.1912;LIX-V:280.
Alfieri E. On genital haemorrhages of newborns. Rendiconti della Associazione Medico-Chirurgica di Parma II; III, 1901.
Ferraresi C. Metrorragia delle neonate [New-borns’ metrorrhagia]. Atti Soc Ital Ostetr Ginecol. 1901;8:51-.
Renouf C. The genitalcrisis and connected manifestations in the fetus and the newborn. Thèse. Paris. 1905.
Rhenter J. The newborngenitalcrisis. The practice of the art of delivering. Paris: Vigot frères. 1928;V:65.
Zubovich VK. Effect of sexual crisis on the state of the newborn in the 1st days of life.Akush Ginekol (Moskwa).1968;44:67-69.
Sacrez R, Levy JM, Muller P, et al. On repercussions of late gestational toxemia on the newborn. Arch Franc Pédiat. 1962;19:360-8.
Rosa P. Sexual endocrinology of the human fetus. Paris: Masson, 1955.
Levitz M, Condon GP, Dancis J, et al. Transfer of estriol and estriol conjugates across the human placenta perfused in situ at midpregnancy. J Clin Endocrinol Metab. 1967;27:1723-9.
Wood CE.. Estrogen in the fetus. Adv Exp Med Biol.2014;814:217-28.
Tulchinsky D, Okada DM. Hormones in human pregnancy. IV. Plasma progesterone. Am J Obstet Gynecol. 1975;121:293-9.
Huber A, Michael S, Feik K. Functional changes in the fetal and infantile endometrium.Arch Gynäk. 1971;211:583-94.
OberWB, BernsteinJ. Observations on the endometrium and ovary in the newborn. Pediatrics. 1955;16:445-60.
Pryse-Davies J, Dewhurst CJ. The development of the ovary and uterus in the foetus, newborn and infant: a morphological and enzyme histochemical study. J Pathol. 1971;103:5-25.
Tietze KW, Intraphuvasak J, Hiersche HD.Functional morphology of the endometrium of the corpus uteri. Arch Gynäkol. 1970;209:331-6.
Hiersche HD, Meinen K. Functional morphology of fetal and infantile endometrial stroma. Arch Gynäkol. 1971;210:164-72.
Kaiser R, Grässel G, Berger-Lang R. Uterine bleeding in newborn girls. Dtsch Med Wochenschr. 1974; 99:1769-71.
Spivack M. Polycystic ovaries in the newborn and early infancy and their relation to the structure of the endometrium. Am J Obstet Gynecol. 1934;27:157-73.
Keller DW, WiestWG, Askin FB, et al. Pseudocorpus luteum insufficiency: a local defect of progesterone action on endometrial stroma. J Clin Endocrinol Metab. 1979;48:127-32.
Chrousos GP, MacLusky NJ, BrandonDD, et al. Progesterone resistance. Adv Exp Med Biol. 1986;196:317-28.
Simpson HW, McArdle CS, Griffiths K, et al. Progesterone resistance in women who have had breast cancer. Br J Obstet Gynaecol. 1998;105:345-51.
Xu Y, Tong J, Ai Z,et al. Epidermal growth factor receptor signaling pathway involved in progestin-resistance of human endometrial carcinoma: In a mouse model. J Obstet Gynaecol Res. 2012;38:1358-66.
Xu W, Zhu S, Zhou Y, et al. Upregulation of mitogen-inducible gene 6 triggers antitumor effect and attenuates progesterone resistance in endometrial carcinoma cells. Cancer Gene Ther. 2014;22:536-41.
Benagiano G, Brosens I. The endometrium in adenomyosis. Womens Health (Lond Engl) 2012;8:301-12.
Taylor HS, Bagot C, Kardana A, et al. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999;14:1328-31.
SavarisRF, Groll JM, Young SL, et al. Progesterone resistance in PCOS endometrium: a microarray analysis in clomiphene citrate-treated and artificial menstrual cycles. J Clin Endocrinol Metab. 2011;96:1737-46.
Brosens I, Benagiano G. Progesterone response in neonatal endometrium is key to future reproductive health in adolescents. Womens Health (Lond Engl). 2016;12:279-82.
Al-Sabbagh M, Lam EW, Brosens JJ. Mechanisms of endometrial progesterone resistance. Mol Cell Endocrinol. 2012;358:208-15.
Salker M, Teklenburg G, Molokhia M, et al. Natural selection of human embryos: impaired decidualization of the endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS One. 2010;5:e10287.
Glatstein IZ, Yeh J. Ontogeny of the estrogen receptor in the human fetal uterus. J Clin Endocrinol Metab. 1995;80:958-64.
Calhoun KC, Padilla-Banks E, Jefferson WN, et al. Bisphenol A exposure alters developmental gene expression in the fetal rhesus macaque uterus. PLoS One. 2014;9:e85894.
Kim SY, Romero R, Tarca AL, et al. Methylome of Fetal and Maternal Monocytes and Macrophages at the Feto-Maternal Interface. Am J Reprod Immunol.2012;68:8-27.
Goldman-Wohl D, Yagel S. NK cells and pre-eclampsia. Reprod BioMed Online. 2008; 16: 227-31
Kammerer U, Rieger L, Kapp M, et al. Immunocompetent cells in the endometrium of fetuses and children. Hum Reprod. 2003;8:969-75.
Terruhn V. A study of impression moulds of the genital tract of female fetuses. Arch Gynecol. 1980;229:207-17.
Fluhmann C. The developmental anatomy of the cervix uteri. Obstet Gynecol. 1960;15:62-9.
Terruhn V. Changes in the shape of the uterine cervix and the development of its epithelium from birth to adolescence. A vaginoscopic study. Arch Gynäkol. 1980a;229:123-36.
Turner G, Coulthard MG. Premenarchal endometrial shedding revealed by peritoneal dialysis. Arch Dis Childhood.1995;73:88–89.
Meng X, Ichim TE, Zhong J,et al. Endometrial regenerative cells: a novel stem cell population. J Transl Med. 2007;5:57.
Lois A, Salamonsen LA. Decidualized Human Endometrial Stromal Cells Are Sensors of Hormone Withdrawal in the Menstrual Inflammatory Cascade. Biol Reprod. 2014;90:1-12.
Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev. 2014;35: 851-905.
Brosens I, Benagiano G. Menstrual preconditioning for the prevention of major obstetrical syndromes in polycystic ovary syndrome.Am J Obstet Gynecol. 2015;213:488-93.
Brosens I, Gargett C, Guo SW,et al. Origins and progression of adolescent endometriosis. Reproductive Sciences. 2016;23:1282-8.
BrosensI, Gargett CC,Gordts S, et al. Neonatal menstruation explains epidemiological links between fetomaternal conditions and adolescent endometriosis. J Endometriosis Pelvic Pain Disord. 2015;7:51-5.
Bianchi P, Benagiano G, Brosens I. The Clinical Diagnosis of Pelvic Endometriosis in Adolescents. MOJ Womens Health. 2017;4:00089.
Puttemans P, Benagiano G, Gargett C,et al. Neonatal Uterine Bleeding as a Biomarker for Reproductive Disorders During Adolescence: a World-Wide Call for Systematic Registration by Nurse-Midwifes. J Mater Fetal Neonatal Med. 2017;30:1434-6.
Received: March 13, 2018;
Accepted: March 27, 2018;
Published: March 30, 2018
To cite this article : Bianchi P, Brosens I, Benagiano G. Neonatal Menstruation and its Meaning. Japan Journal of Medicine. 2018: 1:2.
© 2018 Bianchi P, et al
