Research Article / Open Access
DOI:10.31488/JJM. 166
Clinical Characteristics and Outcomes of 217 Critically Ill Patients with COVID-19: A Retrospective Cohort Study. Outcomes of Critically Ill COVID-19 Patients
José Raimundo A de Azevedo*, Pedro Henrique Dias Brasiliense Frota, Francisco de Souza Trindade Neto, Caroline Marques do Nascimento, Hugo Leonardo de Jesus Gama
*Corresponding author: José Raimundo A de Azevedo, Intensive Care Unit, Hospital São Domingos, Av. Jerônimo de Albuquerque, 540 - Bequimão, São Luís - MA, 65060-645, Brazil, Tel: +55 98 32168110; Fax: +55 98 32168100.
Abstract
Purpose: To describe the clinical characteristics and outcomes of patients with COVID-19 requiring treatment in an intensive care unit. Methods: A retrospective observational study of 217 consecutive patients with a confirmed diagnosis of COVID-19 admitted to a 35-bed ICU at a tertiary hospital in Maranhão, northeaster Brazil was conducted from March 27 to September 30 2020. We collected demographic and clinical laboratory data, as well as data on the therapeutic interventions (including the management of respiratory failure), complications, and mortality. Results: The median age of the 217 patients included in the study was 64 years (IQR 54-75). Overall, 141 patients were male, and 196 had at least one chronic disease, the most common being arterial hypertension (122 patients) and diabetes mellitus (86 patients). Upon ICU arrival, patients had a median PaO2/FIO2 of 188.5 (IQR 125.2 – 300). Fifty-four patients received non-invasive respiratory support, while 163 underwent invasive mechanical ventilation. Seventy-eight patients underwent haemodialysis, and two patients were treated with ECMO. Ninety-two patients died during their ICU stay. Compared to survivors, non-survivors were older and more severely ill, had higher levels of inflammatory markers, required more vasoactive drugs, had a greater need for renal replacement therapy, and had more super infections. Diabetes was the only variable independently associated with ICU mortality. Conclusion: This study suggests that older patients with diabetes are at increased risk of mortality. Non-invasive respiratory support may be essential to disease management.
Key words: COVID-19, SARS-CoV-2, clinical characteristics, critically ill patient, outcomes, intensive care unit
Introduction
Since the first case of infection by the novel SARS-CoV-2 coronavirus in Wuhan in December 2019 [1], more than 120 million cases confirmed by RT-PCR have been registered worldwide and resulted in more than 2,700.000 deaths. The clinical spectrum of COVID-19 ranges from mild to severe, with 5–20% of cases requiring admission to an intensive care unit, and mortality above 50% in patients who required invasive mechanical ventilation and developed multiple organ dysfunction [2].
Some studies have described the clinical characteristics and outcomes of critically ill patients with COVID-19 [2–6]. Although Brazil now has the second highest number of cases and deaths due to COVID-19 worldwide, there is very limited information on the clinical characteristics and outcomes of patients who require treatment in Brazilian intensive care units.
The aim of this study is to describe the clinical characteristics, outcomes, and predictors of ICU mortality of patients with COVID-19 requiring treatment in an intensive care unit in north-eastern Brazil.
Methods
This retrospective observational cohort study was carried out at the Hospital São Domingos, Sao Luis, Brazil, a private tertiary hospital with 370 beds and six intensive care units (ICU) with 64 beds. With the admission of the first patients with COVID-19 on 27 March 2020, we initially made available a 12-bed ICU exclusively for the treatment of patients with COVID-19. At the beginning of April, the number of beds was increased to 35, and immediately afterward, two semi-intensive units (16 and 21 beds) were created for patients in need of non-invasive respiratory support, with the 35-bed ICU admitting only patients in need of advanced respiratory support, translated by PaO2/FIO2 <200, or haemodynamic support with the need to use inotropic/vasoactive drugs. The analysis did not include patients who were admitted to the ICU with a suspected diagnosis of COVID-19 but who had a negative RT-PCR result. Also, patients who were treated in the semi-intensive care units were not included. The study was approved by the Research Ethics Committee of Hospital São Domingos. Due to the study’s observational and retrospective nature, the need for informed consent was waived.
Data were obtained from the hospital’s electronic medical record. Demographic and severity data included age, gender, SAPS 3 score, nutritional risk determined by the Nutritional Risk Screening 2002 (NRS-2002), and comorbidities. Clinical and laboratory data on arrival at the ICU included PaO2/FIO2, blood count, C-reactive protein (CRP), D-dimer, ferritin, fibrinogen, and lactic dehydrogenase (LDH). Drug interventions included the use of hydroxychloroquine, corticosteroids, heparin, and tocilizumab. Data on the characteristics of invasive and non-invasive respiratory support, the use of prone position, extracorporeal membrane oxygenation (ECMO), and vasoactive drugs were also collected. Additionally, the main complications, acute kidney injury requiring haemodialysis, secondary infectious complications, and the outcomes were ascertained.
In our analysis, we defined as non-invasive ventilation patients those who at no time during ICU stay required tracheal intubation and invasive ventilation. Patients initially submitted to non-invasive support who subsequently required invasive ventilation were allocated to the invasive ventilation group, and their data were analysed. For the characterization of acute renal injury, we used the KDIGO score criteria. The presence of an infectious bacterial or fungal complication was determined by the attending physician based on clinical laboratory (mainly procalcitonin), microbiological and imaging data. Serial determination of serum procalcitonin was often crucial to confirm the diagnosis.
The follow-up of the patients was carried out until 31 October 2020.
Statistical analysis
No sample size calculation was performed, the sample size being equal to the number of patients treated during the study period. Statistical analyses were performed using R software version 4.0.2 (R Core Team, 2017). Continuous data are expressed as median and interquartile range [IQR 25–75], Categorical data are expressed as absolute values and/or percentages. Numerical variables were compared between survivors and non-survivors using the Mann-Whitney test. Categorical variables were compared using the chi-square test or Fisher’s exact test.
Survival time was calculated in days, considering date of admission to the ICU as the initial date for all patients. Patients who died, identified as the target event (failure), the final date was date of death. For patients that did not die (censored), the final date was defined as October 31, 2020 or day of hospital discharge. The Kaplan-Meier plot was used to compare mortality during 28-day in- ICU between patients aged 65 and over and those below 65 years. Survival curves were compared using the log-rank test. In all tests, a significance level of 5% was adopted.
A Cox proportional-hazards regression model was adjusted to assess independent risk factors associated with ICU mortality. Initially, univariate analysis was performed, with the following variables: age, sex, SAPS score 3, diabetes, D-dimer, ferritin, PaO2/FIO2, and the use of vasopressors and dialysis. Variables with a p-value ≤ 0.10 in the univariate analysis were included in the multivariate model. Those with a p-value <0.05 remained in the final model (multivariate analysis). To evaluate multicollinearity was calculated the Variance Inflation Factors (VIF). The proportional hazards assumption was checked using statistical tests and graphical diagnostics based on the scaled Schoenfeld residuals.
Results
From 27 March to 30 September 2020, 926 patients were admitted to Hospital São Domingos with a confirmed diagnosis (RT-PCR) of COVID-19. Of this total, 217 patients were treated in the ICU. The median age was 64 years (IQR 54–75), 108 (49.8%) patients were 65 years or older, 141 (64.9%) patients were men, and 187 (86.1%) patients had comorbidities, namely arterial hypertension (122 patients) and diabetes (86 patients). The median duration of the disease before arrival at the hospital was 7 (IQR 4–10) days. In the laboratory evaluation, patients had a median creatinine of 1.05 on arrival at the ICU and markers of inflammation (D-dimer, Ferritin, DLH and fibrinogen) markedly high. Upon arrival to the ICU, PaO2 / FIO2 had a median of 188.5 (IQR 125.2–300).
During their ICU stay, 54 patients (24.8%) were treated exclusively with non-invasive respiratory support including non-rebreathing mask, high flow nasal cannula, and non-invasive positive pressure ventilation, while 163 patients (75.2%) were submitted to invasive mechanical ventilation. Eighty-nine patients (40%) received neuromuscular blocker, 83 patients (38.2%) underwent prone position, and 148 patients (68.2%) received vasopressor drugs. Seventy-eight patients (35.9%) received renal replacement therapy, and two (0.9%) patients were treated with extracorporeal membrane oxygenation (ECMO). Eighty-three patients (38.2%) had bacterial or fungal superinfections. The most used pharmacological intervention was corticosteroids, which were used in 176 patients (81.1%). Heparin for therapeutic purposes was used in 72 patients (33.1%). Hydroxychloroquine was used in 110 patients (50.6%). The use of the drug was concentrated in the months of April and May (100 patients). The median ICU stay was 10 days (IQR 5-17) (Table 1).
Table 1.Clinical characteristics and outcomes of critically ill patients with COVID-19
| All Patients (n=217) | Survivors (n=125) | Non-survivors (n=92) | P Value | |
|---|---|---|---|---|
| Age, y Median (IQR) | 64 (54–75) | 58 (44–68.5) | 72 (63.0–80.7) | <0.0001 |
| Age ≥ 65 years, n (%) | 108 (49,8) | 44 (35,2) | 64 (69,5) | <0.0001 |
| Males, n (%) | 141 (64.9) | 82 (65.5) | 59 (63.0) | |
| SAPS 3, median (IQR) | 59 (49–68) | 54 (42.5–62.5) | 65.5 (58.0–77.0) | <0.0001. |
| Comorbidities Hypertension, n (%) Diabetes, n (%) |
122 (56.2) 86 (39.6) |
63 (49.6) 34 (27.2) |
59 (63.0) 52 (56.5) |
0.1 <0.0001 |
| Symptoms until hospital admission, days Median (IQR) |
7 (4–10) | 8 (5–11) | 5 (3–7) | <0.0001 |
| Laboratory data at ICU admission, White blood cells Creatinine, mg/dL D–dimmer, mg/L Ferritin, ng/ml LDH, U/L Fibrinogen, mg/dL PaO2/FIO2 |
10.200(7.12515.875) 1.05 (0.70–2.20) 1.865 (960–5.480) 1.508 (765–2.630) 463.5 (504.5–751.7) 668.5 (504.5–751.7) 188.5 (125.2–300.0) |
10.200(7.15015.600) 0.91 (0.69–2.29) 1.290 (770–3110) 1.294 (780–1.954) 430 (299–553) 658 (518–770) 220 (129–325) |
10.300(705017.300) 1.3 (0.88–2.2) 2.960 (1.500–7.390) 1.918 (757–3.138) 486 (396–662) 679 (465–706) 177 (110–205) |
0.86 0.0 <0.0001 0.0 0.0 0.59 0.0 |
| Life-sustaining treatment during ICU stay | ||||
| Ventilatory support | ||||
| NIV, n (%) | 54(24.8) | 52(41.6) | 2(2.1) | <0.0001 |
| Invasive ventilation, n (%) | 163(75.2) | 73(58.4) | 90(97.8) | <0.0001 |
| Duration of IMV, d, median (IQR) | 11(7–18) | 11.5 (8.2–20.0) | 10(6–16) | |
| PEEP, cm H2O, median (IQR) | 12(10–14) | 12(11.5–14.0) | 12(10–14) | |
| Prone position, n (%) | 83(38.2) | 39(31.2) | 44(47.8) | 0.0 |
| Neuromuscular blockade, n (%) | 89(41.0) | 43(34.4) | 46(50.1) | |
| Duration, d, median (IQR) | 4.5(3.0–8.0) | 4.0(2.0–8.0) | 5.0(3.0–9.5) | 0.49 |
| Vasopressor, n (%) | 148(68.2) | 62(49.6) | 86(93.4) | < 0.0001 |
| RRT n (%) | 78(35.9) | 24(19.2) | 54(58.6) | < 0.0001 |
| ECMO, n (%) | 2 (0.9) | - | 2 | |
| Superinfection, bacterial, fungal, n (%) | 83(38.2) | 33(26.4) | 50(54.3) | 0<0.0001 |
| Pharmacologic interventions | ||||
| Hydroxychloroquine, n (%) | 110(50.6) | 57(45.6) | 53(57.6) | 0.062 |
| Heparin prophylaxis, n (%) | 135 (62.2) | 84 (67.2) | 51 (55.4) | 0.210 |
| Heparin, therapeutics, n (%) | 72(33.1) | 39(31.2) | 33(35.8) | 0.424 |
| Corticosteroids, n (%) | 176(81.1) | 93(74.4) | 83(90.2) | 0.011 |
| Tocilizumab, n (%) | 11(5.0) | 6(4.8) | 5(5.4) | 0.700 |
| ICU LOS, d median (IQR) | 10(5–17) | 10.0(4.0–16.2) | 11(6.0–17.7) | 0.253 |
| ICU Mortality n (%) | 92(42.4) |
SAPS 3 : Simplified Acute Physiology Score 3; LDH : Lactic dehydrogenase; PaO2/FIO2 : Pressure of Arterial Oxygen to Fractional Inspired Oxygen Concentration; PEEP : Positive end-expiratory pressure; NIV : Non-invasive ventilation; RRT: Renal Replacement Therapy; ECMO: Extracorporeal membrane oxygenation; ICU LOS : Intensive Care Unit length of Stay.
Clinical outcome and risk factors for case fatality
Ninety-two patients died during their stay in the ICU, corresponding to a mortality rate of 42.4%. The length of stay in the ICU was 10 days (5–17) days. In table 1, surviving and non-surviving patients are compared. Non-survivors were older [72 (IQR 63 – 80.7) vs 58 (IQR 44–68.5), p <0.0001] and more severely ill in the SAPS 3 assessment [65.5 (IIQ 58–77) vs 54 (42.5–62.5), p <0.0001]. For 108 patients aged 65 years or older, 44 (35.2%) survived, and 64 (69.5%) deceased (p <0.0001) (Figure 1). It is noteworthy that mortality was significantly higher in diabetics (56.5% vs 27.2%, p <0.0001), but the difference did not reach statistical significance for hypertensive patients. Regarding inflammatory markers, the marker that had the greatest impact as a mortality indicator was the D-dimer [2,960 (IQR 1,500–7,390) vs 1,290 (IQR 770–3,110), p <0.0001], followed by DLH (p = 0.008), and ferritin (p = 0.012). Fibrinogen and leukocyte count had no impact on survival.
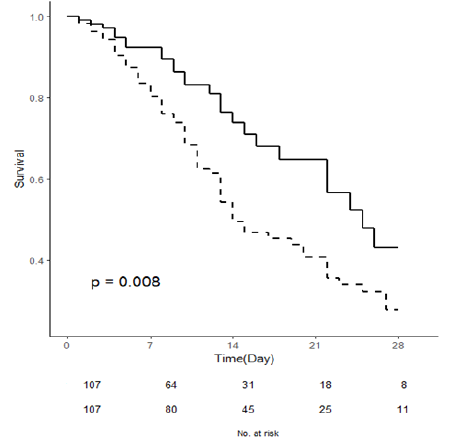
Figure 1:ICU survival curves of patients < 65 (- - - - ) and ≥ 65 years (–)
Regarding life support measures, invasive mechanical ventilation was used as a predictor of mortality, used in 58.4% of survivors and 97.8% of non-survivors (p <0.0001). Vasopressors were used in 93 patients, of whom 4% were non-survivors and 49.6% were survivors (p <0.0001). Dialysis treatment was used in 58.6% of non-survivors and 19.2% of survivors (p <0.0001). Non-invasive ventilation was used in 41.6% of survivors and only 2.1% of non-survivors (p <0.0001). ECMO was used in two patients who did not survive. In total, 54.3% of non-survivors and 26.4% of survivors (p <0.0001) were diagnosed with a bacterial superinfection. No significant difference between the two groups was found in pharmacological therapies, except corticosteroids (93 (74.4%) in survivors vs 83 (90.2%) in non-survivors, p = 0.011).
The Cox proportional hazards model showed that the only variable that was independently associated with ICU mortality was diabetes (HR= 2.0100; p= 0.0256) (Table 2).
Table 2.Univariate and multivariate Cox regression proportional-hazards model of risk factors associated with ICU mortality in COVID-19 patients
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | CI | p Value | HR | CI | p Value | |
| Age | 1.03040 | 1.01-1.05 | <0.001 | 1.0231 | 0.99-1.04 | 0.1031 |
| Male | 094320 | 0.61-1.45 | 0.7910 | |||
| SAPS 3 | 1.02926 | 1.01-1.04 | <0.001 | 1.0015 | 0.98-1.03 | 0.9177 |
| Diabetes | 2.15920 | 1.43-3.27 | <0.001 | 2.0100 | 1.09-2.86 | 0.0256 |
| Hypertension | 1.19580 | 0.78-1.83 | 0.4130 | |||
| D-dimmer | 1.00002 | 1.00-1.01 | 0.0125 | 1.0000 | 1.00-1.01 | 0.1325 |
| Ferritin | 1.00001 | 1.00-1.01 | 0.0686 | 1.0001 | 1.00-1.01 | 0.0929 |
| PaO2/FIO2 | 099799 | 1.00-1.01 | 0.0570 | 0.9973 | 1.00-1.01 | 0.1745 |
| RRT | 1.64420 | 1.08-2.50 | 0.0196 | 0.5964 | 0.52-1.57 | 0.1851 |
| Vasopressors | 3.82060 | 1.66-8.78 | 0.0016 | 2.6173 | 0.93-6.66 | 0.4447 |
HR : Hazard Ratio; CI : Confidence interval; SAPS 3 : Simplified Acute Physiology Score 3; PaO2/FIO2 : Pressure of Arterial Oxygen to Fractional Inspired Oxygen Concentration ; RRT : Renal replacement therapy
Discussion
Among 217 critically ill adults with COVID-19 admitted to a 35-bed ICU of a tertiary hospital in northeast Brazil, the majority were men with hypertension and diabetes; 75.2% of patients received invasive mechanical ventilation, a third received RRT, and the most used pharmacological intervention was corticosteroids, which were used in 176 patients (81.1%). As of 30 September 2020, 42.4% of patients had died in the ICU.
All patients were admitted to the ICU due to acute hypoxemic respiratory failure. Fifty-four patients (24.8%) were treated with non-invasive respiratory support, 163 patients (75.2%) underwent invasive mechanical ventilation, 89 (41%) received a neuromuscular blocker, and 83 patients (38.2%) underwent the prone position. Two patients were treated with ECMO. In a case series of 1591 COVID-19 patients admitted to ICUs in northern Italy, 11% were treated with non-invasive ventilation [3]. Other studies have reported the use of non-invasive ventilation in 14% [7] and 32% of patients [8]. The use of invasive mechanical ventilation in 75.2% of our cases is comparable to that of other studies that enrolled critically ill patients [3,9] but significantly higher than the percentage of other case series that also enrolled critically ill patients [4, 10].
Acute kidney injury is associated with high mortality in patients with COVID-19, especially when renal replacement therapy is required. In these patients, renal injury is related to nonspecific mechanisms, such as the cytokine release and thrombotic events, but also to damage to the renal tubular cell by the virus itself [11]. In our case series, 78 patients (35.9%) underwent renal replacement therapy. Initially, little attention was paid to renal injury in patients with COVID-19. In a case series of non-severe COVID-19 patients, Wang L. et al. [12] found a slight increase in urea and creatinine in 10% of patients. However, this information has not been supported by recent studies. In a study by Yang et al. [4], 29% of patients with severe forms of COVID-19 developed acute kidney injury. Similarly, Diao et al. reported a 27% incidence of AKI [13]. In this study, the incidence of renal injury in patients over 60 years of age was 69.5%.
Mortality due to COVID-19 in the various studies that included ICU patients ranged from 16% [10] to 78% [7]. In this study, the average mortality rate was 42.4% and was significantly higher in patients aged 65 years or older, which is consistent with an earlier study [14]. We did not observe survival differences by sex, but this is inconsistent with the results of a previous study [4]. Compared with survivors, non-survivors presented with more comorbidities, especially diabetes and arterial hypertension, although in the case of hypertension the difference has been shown to be borderline. A previous study revealed that original comorbidities were potential risk factors [4]. Non-survivors also showed expressively higher inflammatory markers, especially d-dimer, LDH, and ferritin, corroborating other studies [6, 15]. Also, mortality was substantially higher in patients undergoing invasive mechanical ventilation, receiving vasopressors, and in those undergoing renal replacement therapy.
The incidence of bacterial or fungal superinfection in general has either not been addressed or very superficially addressed by the various studies that analysed clinical characteristics and outcomes in patients with COVID-19 [2, 9, 19]. A prospective study involving 257 critically ill patients reported that the incidence of superinfection was unknown and that most patients received antimicrobials empirically [6]. A retrospective study involving 239 critically ill patients in Wuhan reports a reduced incidence of bacterial superinfection with no impact on mortality [5]. In this study, 83 (38.2%) patients had a bacterial or fungal superinfection, and the incidence was significantly higher in non-survivors.
Regarding drug interventions, the only intervention that resulted in a significant difference between survivors and non-survivors was corticosteroids, used in 90.2% of non-survivors and in 74.4% of survivors (p <0.011). A preliminary report compared the use of dexamethasone (6 mg/day) with the usual treatment and showed a 28-day mortality reduction in patients with COVID-19 submitted to invasive and non-invasive respiratory support [16]. Since the publication of that study, the use of dexamethasone at a dose of 6mg/day has been incorporated into WHO guidelines [17] and become widely used, although the study left some unanswered questions, such as the large number of exclusions without a clear explanation [18]. No significant difference was found between survivors and non-survivors in relation to the other drugs used.
In this cohort of patients, 86.1% had at least 1 comorbidity, higher than that reported by Grasselli et al. (68%) [3] and other studies [1, 8, 10]. Similar to previous reports [8, 10], hypertension was the most common comorbidity, followed by diabetes. Logistic regression analysis showed that hypertension did not impact mortality, and diabetes was the only variable independently associated with ICU mortality. Two recently published studies identified diabetes as an independent determinant of hospital mortality in patients with COVID-19 [20, 21].
This study has some limitations. Firstly, it is a retrospective study, which limits the volume and quality of information that can be obtained in prospective studies. Secondly, it was carried out in a single hospital that only serves patients using health insurance, potentially limiting the generalizability of the results beyond the socioeconomic reality of the population studied. Thirdly, our analysis incorporated data collected until 30 September 2020. The condition of patients who remained in the ICU was monitored until 31 October.
Conclusions
This single-centre study suggests that older patients with diabetes are at increased risk of mortality. Non-invasive respiratory support (high flow nasal cannula oxygen) may be essential to disease management.
Highlights
The study findings implicate that older patients with diabetes are at increased risk of mortality. Non-invasive respiratory support and the monitoring of inflammatory markers may be crucial to disease management.
Study Registrations
Research Ethics Committee of Hospital São Domingos: Approval number 4.213.523.
ClinicalTrials.gov Identifier: NCT04454372
Acknowledgements
Not applicable
Funding
Not applicable
Compliance with ethical standards
Conflicts of Interest
The authors declare no competing interests
Ethical Approval
Included in methods
Availability of data and material: In the title page
Author Contributions
JRAA was responsible for study design, data analysis, statistical analysis, and critical revision of the manuscript for important intellectual content. PHDBF contributed to the study design, coordination of data acquisition, interpretation of the data and critical revision of the manuscript for important intellectual content. FSTN contributed to the study design, acquisitions of the data, and interpretation 206 of the results. CMN contributed to acquisition of the data, interpretation of the result. HLJG contributed to the study design, acquisitions of the data, and interpretation 206 of the results.
References
1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395(10223):497–506.
2. Xie j, Wu J, Li S, et al. Clinical characteristics and outcomes of critically ill patients with novel coronavirus infectious disease (COVID-19) in China: a retrospective multicenter study. Intensive Care Med. 2020; 46(10):1863–1872.
3. Grasselli G, Zangrillo A, Zanella A, et al. COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020; 323(16):1574–1581.
4. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020; 8:475–481.
5. Xu j, Yang X, Yang L, et al. Clinical course and predictors of 60-day mortality in 239 critically ill patients with COVID-19: a multicenter retrospective study from Wuhan, China. Crit Care. 2020; 24(1):394.
6. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020; 395(10239):1763–1770.
7. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020; 395(10229):1054–1062.
8. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 382(18):1708–1720.
9. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020; 323(20):2052–2059.
10. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020; 323(11):1061–1069.
11. Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020; 98(1):219–227
12. Wang L, Li X, Chen H, et al. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol. 2020; 51(5):343–348.
13. Diao B, Wang CH, Wang RS, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. medRxiv. 2020; 10.1101/2020.03.04.20031120.
14. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020; 323(13):1239–1242.
15. Wang Y, Lu X, Li Y, et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. 2020; 201(11):1430–1434.
16. Horby P, Lim WS, Emberson J, et al. Dexamethasone in hospitalized patients with Covid-19. Preliminary report. N Engl J Med. 2020; NEJMoa20211436.
17. Rochwerg B, Agoritsas T, Lamontagne F, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020; 370:m3379.
18. Dexamethasone in hospitalized patients with COVID-19: addressing uncertainties. Lancet Respir Med. 2020; 8(12):1170–1172.
19. Fadel FA, Al-Jaghbeer M, Kumar S, et al. Clinical characteristics and outcomes of critically Ill patients with COVID-19 in Northeast Ohio: low mortality and length of stay. Acute Crit Care. 2020; 35(4):242–248.
20. Baron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19- related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020; 8(10):813–22.
21. Miller LE, Bhattacharyya R, Miller A. Diabetes mellitus increases the risk of hospital mortality in patients with Covid-19: Systematic review with meta-analysis. Medicine (Baltimore). 2020; 99(40):e22439.
Received: June 08, 2021.
Accepted: June 14, 2021.
Published: June 16, 2021 .
To cite this article : de Azevedo JRA , Frota PHDB, Neto FDST, et al. Clinical Characteristics and Outcomes of 217 Critically Ill Patients with COVID-19: A Retrospective Cohort Study. Outcomes of Critically Ill COVID-19 Patients. Japan Journal of Medicine. 2021; 4:1.
© 2021 de Azevedo JRA, et al.
