Mini Review / Open Access
DOI: 10.31488/jjm.1000117
Colon capsule endoscopy for colorectal cancer screening in patients with the positive fecal occult blood test (FOBT) in Japan
KonosukeNakaji*
Endoscopy Center, AishinkaiNakae Hospital, Wakayama, Japan
*Corresponding author:KonosukeNakaji, MD, FACP, Endoscopy Center, AishikaiNakae Hospital, 30-1 Funadokoro Wakayama 640-8461, Tel:+81-73-451-0222; Fax: +81-73-541-0837;
Abstract
Screening for colorectal cancer (CRC) has been shown to be effective in reducing mortality from the disease. Unfortunately, however,while the decrease in CRC mortality is mainlydue to the contribution of the recommended colonoscopy, its acceptability is still low. Therefore, less-invasive screening modalities with equivalent sensitivity and specificity for the detection of polyps and cancer are desired. Colon capsule endoscopy (CCE) was first introduced in 2006 as a wireless, non-invasive technique for imaging of the large bowel that does not require sedation or X-ray exposers. In this review, the current role of CCE for patients with the positive fecal occult blood test (FOBT) in Japan will be discussed.
Keywords: colon capsule endoscopy, colorectal cancer screening, positive fecal occult blood test (FOBT)
Introduction
Colorectal cancer is the leading cause of death worldwide [1], and increased incidence in the Japanese population [2] with Westernized-Japanese diet habits. Today, it is the first leading cause of cancer deaths in women and the second for both men and women in Japan[3]. Japanese CRC screening programs for colorectal cancer based on fecal occult blood testing (FOBT) started in 1992. It is an annual two-day FOBT method, and offeredto those 40 years of age and over, and a total colonoscopy is performed for those with at least one positive FOBT [4]. There has been sufficient evidence of colorectal cancer screening programs using FOBT and colonoscopy reducing the motility of CRC[5]. However, participation rates are only 41.4% for men and 34.5% for women in Japan so far[6]. There may be several reasons therecommended colonoscopy is refused, including perceptions of discomfort, pain, or being worried about anal intubations especially for women, and the invasive nature of colonoscopies that include perforations and bleeding. Recently, the second-generation colon capsule endoscopy (CCE) (PillCam Colon 2: Medtronic Minneapolis, USA) began to be covered by Japanese health insurance. It is an easy and non-invasive method for screening colorectal cancer andonly involves swallowing a capsule. Thus, this newer CCE is thought to be useful for detecting colorectal lesions in patients with FOBT who are unsuitable or unwilling to undergo acolonoscopy. However, to our knowledge, there have been no reports so far on the role of CCE in the FOBT-based Japanese population cohort. Therefore, in this article, we will review the current works of literatureand presentour trials on this issue in Japan.
Technical characteristics, indications, and cost-effectiveness
The PillCam COLON2 capsule (Medtronic, Minneapolis USA) (Figure 1), which records digital images on 2 camera heads(31mm×11 mm,weight 2.9 g) with wide visualization (172° for each camera; 360°for both)at a rate ranging from 4 to 35 frames per second in accordance with the speed of the capsule,whichhas an adaptive frame rate (AFR) and an approximately 10-hour procedure time,was used. The images were transmitted to a recording device (DR3) wirelessly carried by the patient with sensorsattached to the patient’s skin(Figure2). The data were transferred from the device to a computer that uses software (RAPID v.8.3 now available)(Figure 3) to compile a video thatwas then analyzed by a board-certified technical assistant and later confirmedby a board-certified gastroenterologist. We sometimes found that only one thumbnail (picture) represented an important finding, so a training system for CCE is necessary. Several reader education programsfor technical assistants and gastroenterologists such as computer-based learning (E-learning) and hands-on seminarshave been reported in both Europe and Japan [7] [8]. In Japan, the indication for CCE is either a failed total colonoscopy or the presumption that a colonoscopy would be difficult to conduct due to adhesion after an operation on the abdomen and so on.We use patency capsules (a soluble dummy capsule for small bowel capsule endoscopies) forsome patients if apatient isworried about retention. Contraindications of CCE are almost the same aswith the small bowel capsule,includingpatients with intestinal obstructions, dysphasia, implanted electric devices, and confirmed or suspected pregnancy.CCE examination has been estimated to cost approximately between ¥10,000 and ¥30,000 with medical insurance in Japan. At our institute, all CCE has been performed in an outpatient setting because it was not covered by Japanese social insurance in the inpatient unit.One study described the introduction of CCE to CRC screening in Japan showed it to be more cost-effective than conventional screening (20% or more) with the quality–adjusted life years using the Markov model [9].

Figure 1.PillCam Colon 2 capsule
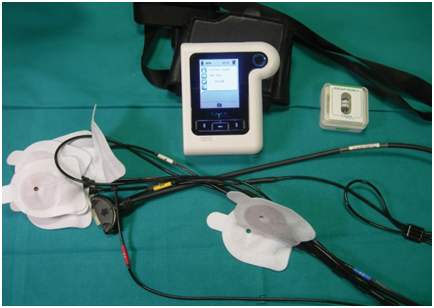
Figure 2.DR3 and sensors
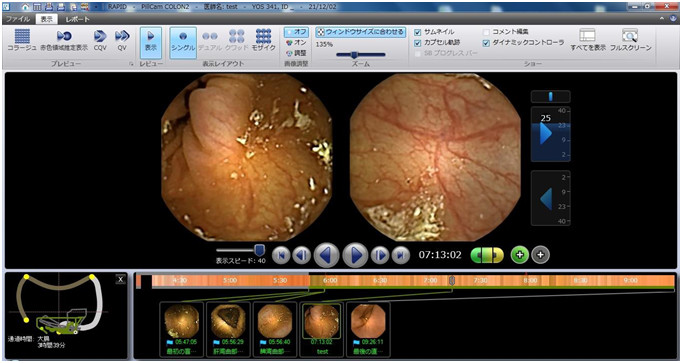
Figure 3.Rapid for PillCam software R8.3
Bowel preparation for CCE
Unlike conventional colonoscopy, adequate bowel preparation is essential toobtaining an accurate CCEdiagnosis because acleaning procedure is not available.Table 1 shows a recent bowel preparation regimen we did for CCE. In summary, the day before the examination, the patient followed a low-residue diet and ingested a hypertonic magnesium citrate solution. On the day of the examination, we usedpolyethylene glycol that contained ascorbic acid (Moviprep: EA pharma, Tokyo, Japan) as both the preparation and the booster with 10mg of metoclopramide weregiven intravenously after capsule ingestion.Patients are then required to walk up and down stairs during the examination in the hospital until they excrete the capsule to accelerate bowel movements [10].A glycerin enema was used in cases of delayed excretion. In our prospective and multicenter (9 institutes in Japan) study of this regimen (n=82, 45male patients, average ageof60.0±13.8years old), the capsule was excreted 85% of the time and excellent visualization of the entire colon wasachieved 82% of the time.The average colon transit time was 172±95minutes and the average total water intake was3,605±518mL.A glycerin enema was used in 33% of the cases.A sodium phosphate solution is commonly used as a booster in Europe [11] but has limited use in the US and is not available in Japan due to concernsaboutrenal toxicity. Several boosters and prokinetics for CCE have been tried such asGastrografin (a contrast medium for the digestive tract) [12], Daikenchuto(Tsumura, Tokyo,Japan: a Japanese herbal medicine) [13],Neostigmine (0.25mg intramuscularadministration) [10]in Japan,and the Japanese association for capsule endoscopy recently recommended aromatic castor oil as a prokinetic [14]. Linaclotide, which activates the cell surface receptors of guanylatecyclase 2C (GC-C), may also act as a prokinetic. Our recent study showed the colon transit time was significantly shorter in the Linaclotide(L) group(n=11) than in the non-Linaclotide(NL) group (n=37) (P<0.001). The fluid intake was significantly smaller in the L group than in the NL group(P<0.001). The acceptability was significantly higher in the L group than in the NL group (P=0.04). Also, in our transit times study for CCE, male patients, exercising during CCE, and patients who do not have diabetes mellitus showed a significantlyshorter colon transit time[10].Despite these efforts, an ideal preparation method that has asufficient cleansing level, high excretion ratio, and is well-tolerated by CCEpatients has yet to be determined.
Table 1. Bowel preparation regimen with ascorbic acid containing polyethylene glycol (Moviprep) for colon capsule endoscopy.
| Day | Time | Action | Dose of Solution | Total amount of solution |
|---|---|---|---|---|
| Day before exam | Noon, Evening, Between these meals |
Low- residue diet | ||
| Evening | Magcorol P hypertonic solution 180 mL | 180mL | ||
| Bedtime | Laxoberon | 10mL | ||
| Day of exam | Wake up | Mosapride 20mg | ||
| -2hrs before capsule ingestion | Moviprep 1200mL Water 600mL |
1800mL | 1800mL | |
| Capsule Ingestion Metoclopramide 10mg IV |
200mL | 2000mL | ||
| Reaching small Bowel | Magcorol P isotonic solution 900mL | 900mL | 2900mL | |
| +1 hrs, from reaching small bowel | Moviprep 400mL Dimethicone solution 200mL |
600mL | 3500mL | |
| +2 hrs, from reaching small bowel or reaching colon | Moviprep 400mL water 200mL |
600mL | 4100mL | |
| +1 hrs, from reaching colon | Light meal | |||
| +3 hrs from reaching colon or 16:00 | Glycerine enema 120mL |
Moviprep: polyethylene glycol + ascorbic acid; Lacobaron: sodium piorate hydrate ; Magcorol P: magnesium citrate
CCE for detection of colon polyps
The first-generation colon capsule, which was released in 2006, had limited sensitivity(64 %)and specificity(84%) for detecting colon polyps (≥6 mm)[15]. In addition, sensitivity and specificity for advanced adenoma were 73% and 79%, respectively [15]. However, with the newer second-generation colon capsule, which provides AFR with wider visualization as mentioned above, the sensitivity and specificity have increased[16-21], and one recent study showed(n=50)for≥6 mm polypsit was93% sensitivity and 80%specificity and ≥10 mmit was 100% sensitivity and 100% specificity [21]. A multicenter study in Japanheld in 2105 (n=66) showed the sensitivity for clinically significant polyps(≥6mm) was 94% with the second-generation CCE[22].A study on 165 Japanese patients for whom the second-generation capsule was used for polyp detection between Nov. 2013 and Dec. 2017 in our institute for significant polyps (>6mm) showed the positive predictive value (PPV)was 93%as compared to colonoscopy. As mentioned above, the sensitivity and specificity of the second generation CCE for polyp detection have improved remarkably compared with that of the first generation, which led simultaneously to its approval by the U.S. Food and Drug Administration and coverage by Japanese health insurance in 2014.In addition, CCE has been adopted as an option for screening CRC every 5 years by the U.S.Preventive Services Task Force. Furthermore, second-generation CCE may detect small polyps(<5 mm) or sessile polyps better comparedto other modalities; CS/CTC due to no inflation of the intestinal lumen [23].
CCE for colorectal cancer screening in patients with the positive fecal occult blood test (FOBT)
To the best of our knowledge, only a few trials have been conducted evaluating second-generation CCE in patients with positive FOBT. In 2014, Grainne Holleran et al. [24] of Ireland reported that a colonoscopy followedby CCE the next day for patients who are FOBT positive (n=62) detected at least one polyp in 58 %, significant lesions in 29 %, and cancer in 2 % of them. The negative-predictive value of CCE was high both for any polyps (90 %) and significant lesions (96 %). They concluded there was a strong correlation between CCE and colonoscopy for any lesions and significant lesions. In 2104, Rondonotti[25]of Italy reported comparisons of CCE and CTcolonography (CTC) for positive FOBT patients. CCE identified the polyps with 88.2%sensitivity and 87.8% specificity. CTC identified the polyps with 88.2%sensitivity and 84.8% specificity. They indicated thatCCE seems better tolerated than CTC, and CCE could be a reliable alternative to CTC for positive FOBT. In 2018, Mathieu Pioche et al. [26]of France also compared CCE and CTC on positive FOBT patients, and reported that the 84% of CCE patients (n=16/19) were positive. CCE detected 60% of potentially neoplastic lesions(6 adenomas or cancers, 6 clinically significant polyps). On the other hand, CTC detected positive findings of 46.4 %(13/28), and 8 potentially neoplastic lesions (28.6%). They concluded there were more potentially neoplastic lesions diagnosed in the CCE group than in the CTC group (p=0.04).We enrolled 35 Japanese patients (15 males, 20 females, with a mean age of 61.6±12.8 years old) with an asymptomatic positive FOBT resultat three institutes in a cohort study. It was assumed that thirty-one patients would have difficulty with a conventional colonoscopy due to colonic adhesion as the result of previous abdominal surgery. Our study showed that the positive rate was 94.3% with a completion rate of 86.6%. Of these, 40 polyp lesions were detected (an average of 1.1 lesions in each case). The average water intake was 3,706±684 mL, the average colon transit time was 164.5±88.3 min.,and the adequate cleansing rate including excellent and good was 71.4%. Subsequently, 21 significant lesions were endoscopically resected within 3months of the CCE. There were 15 adenomas, 3 hyperplastic lesions, and 3 cancerous ones (1advanced and 2early) (Figure 4,5a and 5b). The colon transit time was significantly shorter and the liquid intakewas significantly smaller (P<0.001)in positive FOBT patients than those of the other indications of 130 patients. There were no adverse events. Based on these results with theirreasonable predictive value, safety, and good acceptability, CCEcould be a suitable alternative to colonoscopy for patients with positive FOBT in whom it is not technically feasible to undergo a conventional colonoscopy in the Japanese colorectal cancer screening program.We propose a newscreening algorithm for the CRC program using CCE as shown Figure 6.
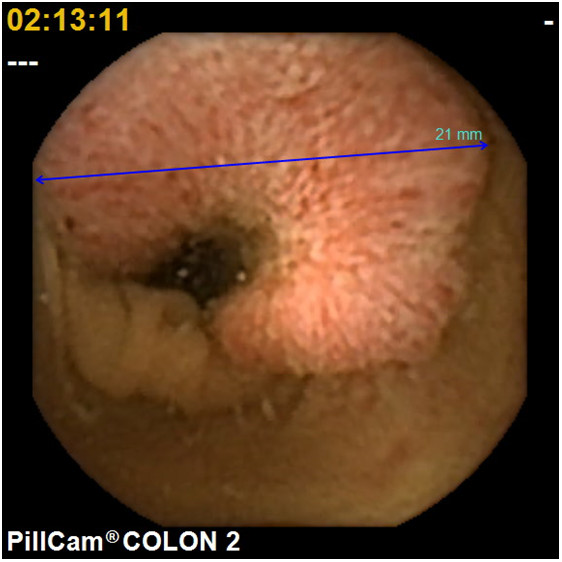
Figure 4.An advanced colonic cancer with positive FOBT patients detected by second-generation colon capsule endoscopy
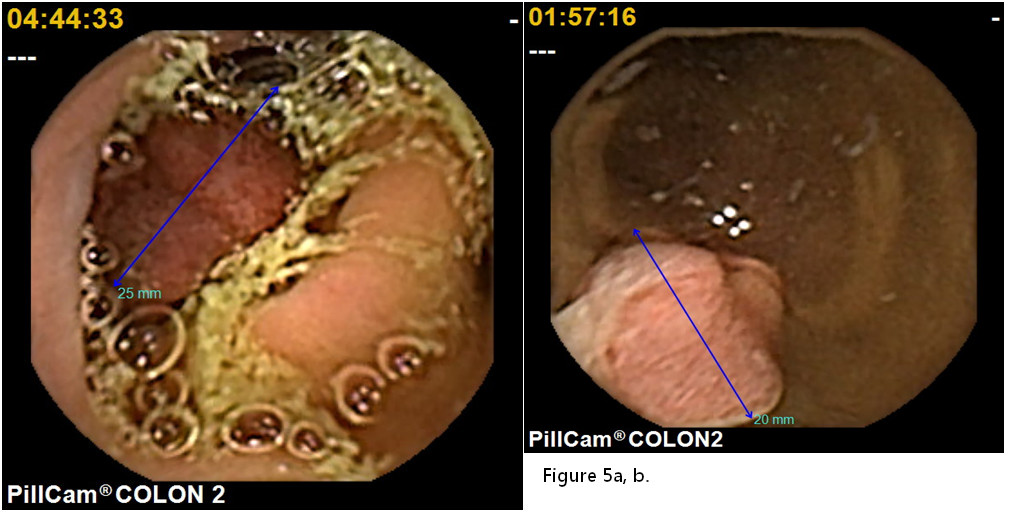
Figure 5a,b.An early colonic cancer with positive FOBT patients found by second-generation colon capsule endoscopy.
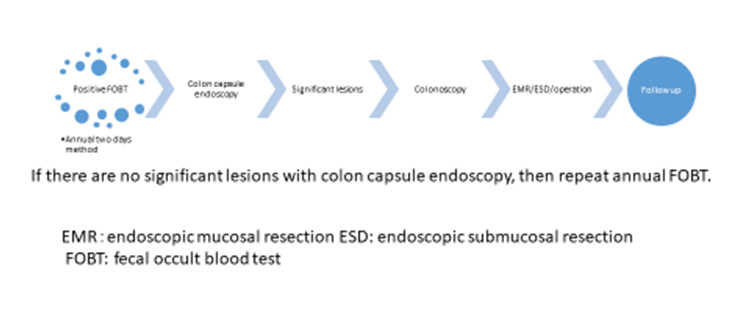
Figure 6.Algorism for colorectal cancer screening with colon capsule endoscopy.
Future perspectives for CCE
With participation in the colorectal screening program increasing with FOBT,an increase in thenumber of colonoscopiesforthese patients is inevitable. However, today, there are too fewcolonoscopiststo conductthe screening colonoscopies for these patients especially in rural areas in Japan. To compensate for this, CCE will play an important role in detecting CRC. With improving device technology such as detection of the top 100 significant lesionswithnewer software, CCE will be a more solidmodality that is used to evaluateCRC. Further prospective randomized studies with large numbers of subjects are necessary to establish the role of CCE in diagnostic algorithms for colorectal cancer screening for FOBT positive patients.
Disclosure Statement
There are no conflicts of interest to declare.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2015; 65:5-29.
Jang E, Chung DC. Hereditary Colon Cancer: Lynch Syndrome. Gut and Liver. 2010; 4:151-160.
Katanoda K, Hori M, Matsuda T, et al. An updated report on the trends in cancer incidence and mortality in Japan, 1958-2013. Jpn J ClinOncol. 2015; 45:390-401.
Sano Y, Byeon JS, Li XB et al. Colorectal cancer screening of the general population in East Asia.Dig endosc. 2106; 28:243-249.
Sekiguchi M, Matsuda T, Saito Y.What is the optimal colorectal cancer screening program for an average-risk population? TranslGastroenterolHepatol. 2017; 2:17.
Navarro M, Nicolas A, Ferrandez A, et al. Colorectal cancer population screening programs worldwide in 2016: An update. World J Gastroenterol.2017; 23:3632-3642.
Fernandez UI, Panter S, Carretero C, et al. International core curriculum for capsule endoscopy training courses. Endoscopy International Open.2017; 05: E526-E538.
Watabe H, Nakamura T, Yamada A, et al. Assessment of an electronic learning system for colon capsule endoscopy: a pilot study. J Gastroenterol.2016; 51:579- 585.
Sakamaki H, Tajiri H, Inoue S, et al. Cost-effectiveness analysis of capsule endoscopy in screening for colorectal cancer in Japan.Value in health. 2013; 16: A323-A636 A414.
Nakaji K, Suzumura S, Nakae Y et al. Clinical factors associated with intestinal transit time of colon capsule endoscopy.JWakayama Med Soc. 2016; 67:96-100.
Spada C, Hassan C, Galmiche JP et al. Colon capsule endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2012; 44(5):527-536.
Togashi K, Fujita T, Utano K, et al. Gastrografin as an alternative booster to sodium phosphate in colon capsule endoscopy: safety and efficacy pilot study. EndoscInt Open. 2015; 3:E659-661.
Nakaji K, Suzumura S, Fujita A, et al. Effectiveness of Daikenchuto, a Traditional Japanese Herbal Medicine, in Accelerating Capsule Endoscopy Transit Time-A Prospective Pilot Study. New Tech Gastroint Endo.2011.
Hotta N. The Use of Castor Oil for Bowel Preparation for Colon Capsule Endoscopy Open Journal of Medical Imaging.2016; 6:103-107.
Van Gossum A, Munoz-Navas M, Fernandez-Urien I, et al. “Capsule endoscopy versus colonoscopy for the detection of polyps and cancer,” N Engl J Med.2009; 361: 264-270.
Eliakim R, Yassin K, Niv Y, et al. Prospective multicenter performance evaluation of the second-generation colon capsule compared with colonoscopy.2009;Endoscopy. 41:1026-1031.
Spada C, Hassan C, Munoz-Navas M, et al. Second-generation colon capsule endoscopy compared with colonoscopy. 2011; GastrointestEndosc. 74: 581-589 e1.
Hagel A.F., Gäbelee E, Raithel M, et al. Colon capsule endoscopy: detection of colonicpolyps compared with conventional colonoscopy and visualization of extracolonic pathologies. 2014; Can J GastroenterolHepatol.28: 77-82.
Holleran G, Leen R, O’Morain C, et al. Colon capsule endoscopy as possible filter test for colonoscopy selection in a screening population with positive fecal immunology.Endoscopy.2014; 46:473-478.
Rex DK, Adler SN, Aisenberg J, et al. Accuracy of capsule colonoscopy in detecting colorectal polyps in a screening population. Gastroenterol. 2015; 148: 948.e2-967.e2.
Akyüz Ü, Yılmaz Y., İnce AT, et al.Diagnostic role of colon capsule endoscopy in patients with optimal colon cleaning. GastroenterolResPract.2016; Article ID 2738208, 5 pages.
Saito Y, Saito S, Oka S, et al. Evaluation of the clinical efficacy of colon capsule endoscopy in the detection of lesions of the colon: prospective, multicenter, open study. GastrointestEndosc. 2015 82:861-869.
Spada C, Hassan C, Barbaro B, et al. Colon capsule versus CT colonography in patients with incomplete colonoscopy: a prospective, comparative trial. Gut.2015; 64: 272-281.
Holleran G, Leen R, O’Morain C, et al.Colon capsule endoscopy as possible filter test for colonoscopy selection in a screening population with positive fecal immunology. Endoscopy.2014; 46:473-478.
Rondonotti E, Borghi C, Mandelli G, et al. Accuracy of capsule colonography and computed tomographic colonography in individuals with positive results from the fecal occult blood test. ClinGastroenterolHepatol. 2014; 12:1303.
Pioche M, Ganne C, Gincul R, et al. Colon capsule versus computed tomography colonography for colorectal cancer screening in patients with positive fecal occult blood test who refuse colonoscopy: a randomized trial. Endoscopy. 2018.
Received: June 10, 2018;
Accepted: June 23, 2018;
Published: June 27, 2018
To cite this article : Nakaji K. Colon capsule endoscopy for colorectal cancer screening in patients with the positive fecal occult blood test (FOBT) in Japan. Japan Journal of Medicine. 2018: 1:4.
© Nakaji K. 2018.
