Research Article / Open Access
DOI: 10.31488/jjm.1000102
Interface Pressures Derived from Oversized Compression Arm Sleeves
Kotaro Suehiro, NoriyasuMorikage, Koshiro Ueda, Makoto Samura, Yuriko Takeuchi,Takashi Nagase, Takahiro Mizoguchi,Kimikazu Hamano
Division of Vascular Surgery, Department of Surgery and Clinical Science, Yamaguchi University Graduate School of Medicine, Yamaguchi, Japan
*Corresponding author:Kotaro Suehiro, Minamikogushi, Ube, Yamaguchi 755-8505, Japan, Tel: +81-836-22-2260; Fax: +81-836-22-2423;
Abstract
Objectives: To clarify interface pressures (IP) derived from oversized compression sleeves. Methods: Twenty healthy female volunteers whose left arms fit size S wore compression sleeves of sizes S, M, L, and LL. IPs on the forearm and upper arm were measured with each sleeve on. Results: The median IP was 24 (range; 18–30) mmHg on the forearm and 21 (15–32) mmHg on the upper arm using S-sized sleeves. These decreased to 19 (14–24) and 17 (11–24) mmHg (p < 0.05 vs. S-sized, each) for M-sized; 17 (10–22) and 16 (12–22) mmHg (p < 0.05 vs. S-sized, each) for L-sized; and 13 (6–21) and 10 (7–18) mmHg (p < 0.05 vs. S-sized, each) for LL-sized sleeves, respectively. The number of participants in whom IP > 20 mmHg was maintained on the forearm was 19 (95%) with an S-sized sleeve, 9 (45%) with M-sized, 7 (35%) with L-sized, and 2 (10%) with LL-sized. The significant difference in IPs on the upper arm and forearm was maintained only with S-sized sleeves.: Only a compression sleeve of appropriate size could stably provide IPs at the therapeutic range and the designated graduation of IP.
Keywords: compression therapy; arm sleeve; lymphedemaa
Introduction
Compression therapy using an elastic arm sleeve is the mainstay in conservative therapy for arm lymphedema (LE) [1]. Although the dose-response relationship between compression pressure and edema reduction has not yet been clarified in arm LE,an interface pressure (IP) above 20 mmHg is generally considered to be optimal [2-5]. This is a milder compression compared to that derived from compression stockings for leg LE or chronic venous insufficiency, in which an IP above 30 mmHg is recommended [6]. However, arm compression sleeves are still too tight and resistant for weak, elderly, or handicapped people to apply by themselves using the single contra lateral hand. For these cases, prescribing larger-sized sleeves may be the choice. We previously reported that knee-high round-knitted compression stockings that were 1 to 2 sizes too large could provide comparable IPs to those provided by an appropriate size stocking [7].We also reported that, although less effective, a reasonable edema reduction could be achieved using such oversized stockings,while maintaining much better adherence [8]. However, IPs derived from oversized compression arm sleeves have never been reported precisely.In this study, we investigated such IPs and discussed whether they could be applicable for the treatment of arm lymphedema.
Patients and Methods
This study was approved by the Institutional Review Board of Yamaguchi University Hospital (Ube, Yamaguchi, Japan). All participants provided signed, informed consent before enrollment.The compression sleeves evaluated in this studywere ready-made round-knitted arm sleeves (Medical Support ® arm sleeve, Medicks Corporation, Tokushima, Japan), which are supposed to provide 15–25 mmHg at the wrist. The study subjects were 20 healthy female volunteers with amedian age of 34 (range 22–61) years, whose left arms were fitted as an S-sized sleeve according to the manufacturer’s brochure. The characteristics of the participants’ arms and the recommended sizes listed in the brochure are summarized in Table1. There were significant overlaps among the definitions of arm sizes, but the participants were selected based on their wrist circumference.
Each participant first put on anS-sizedsleeve, then changed into anM, L, and LL-sizedsleeve, in that order. With the participant in the sitting position, the IP under each sleeve was measured with the arm in the horizontal position and then in the dependent position using an air pack-type analyzer (Model AMI-3037-SB, AMI Co., Tokyo, Japan). The sensor was placed on theventral surface of the middle of the forearm and the middle of the upper arm (Figure 1).
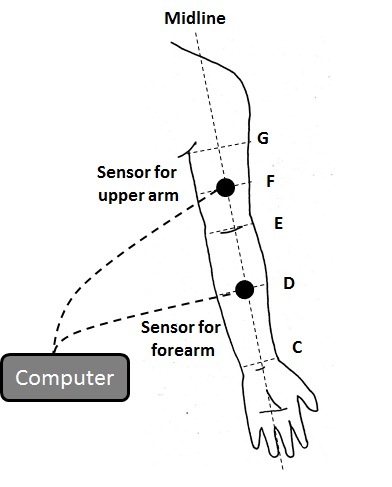
Figure 1.The positions of sensors and the points of circumference measurements.C: the level of the wrist; D: middle of C and E; E: the level of the cubital fossa; F: the middle of E and G; G: the level of the axilla.
Statistical analysis
The results are expressed as the median (range) or count, unless otherwise indicated. The Wilcoxon signed rank sum test was used to test the differences in IPs obtained by each sleeve, and to test the differences in IPs obtained based ondifferent limb positions. The Mann-Whitney U-test was used to test the differences in IPs on the upper arm and forearm. Statistical analyses were performed using JMP 11.0 (SAS Institute, Cary, NC, USA). A p-value < 0.05 was considered significant
Results
Figure 2 demonstrates the IPs obtained from each sleeve worn by the participants.With the arm in the dependent position, a median IP of 24 (range, 18–30) mmHg was recorded at the middle of the forearm, and21 (15–32) mmHg at the middle of the upper arm using an appropriately sized, i.e. S-sized, sleeve.These values decreased to 19 (14–24) mmHg (p< 0.05 vs. S sleeve)and 17 (11–24) mmHg (N = 18; p< 0.05 vs. S sleeve),respectively, for an M-sized; and to 17 (10–22) mmHg (p< 0.05 vs. S sleeve) and 16 (12–22) mmHg (N = 14; p< 0.05 vs. S sleeve) for an L-sized; and finally, to13 (6–21) mmHg (p< 0.05 vs. S sleeve) and 10 (7–18) mmHg (N = 9; p< 0.05 vs. S sleeve) for an LL-sized. The oversized sleeves did not properly fit the upper arm in a certain number of participants (M-sized: 2, L-sized: 6, LL-sized: 11), so measurement of IP could not be performed in these cases.
The number of participants in whom IP > 20 mmHg was maintained on the forearm were 19 (95%) with an S-sized sleeve, 9 (45%) with M-sized, 7 (35%) with L-sized, and 2 (10%) with LL-sized. The significant difference in IPs on the upper arm and forearm was maintained only when an S-sized sleeve was used, whereas no significant differences were observed when using oversized sleeves.
Figure 3 demonstrates the change in IPs on the forearm based on the arm position.Using S-, M-, and L-sized sleeves, a small but significant increase in IP was observed when the arm was in a dependent position(S-sized: 1 [-1–3] mmHg, M-sized: 1 [-2–3] mmHg, L-sized: 0.5 [-1–3] mmHg). However, this difference was not observed when using the LL-sized sleeve(-1 [-3–2] mmHg).
Discussion
In contrast to oversized knee-high compression stockings, which were able to maintain comparable IPs even when they wereup to 2 sizes too large (one at the level of the transition of the gastrocnemius muscle into the Achilles tendon [B1 level]), IPs, both on the middle of the upper arm and forearm, were significantly decreased with compression arm sleeves that were even 1-size too large. As a result, IP > 20 mmHg could not be maintained using oversized sleeves. Moreover, the designated graduated compression could not be achieved with oversized compression sleeves.
The purpose of compression therapy, particularly in the treatment of LE, is 1) to increase interstitial pressure to reduce capillary filtration,2) to improve venous and lymphatic drainage, and 3) to enhance muscle pump function [9]. In the arm with LE, increased capillary filtration was reported because of systemically increased vascular endothelial growth factor C concentration and locally increased mono cyte chemotactic protein 1 concentration, suggesting the presence of low grade inflammation [10].
Table 1. Participants’ arm characteristics (N = 20)
| Circumference | Median (range) | Recommended size (cm) |
|---|---|---|
| The level of the wrist (C; cm; median [range])The middle of forearm (D; cm; median [range])
The level of the cubital fossa (E; cm; median [range]) The middle of the upper arm (F; cm; median [range]) The level of the axilla (G; cm; median [range]) Distance C-E (cm; median [range]) C-G (cm; median [range]) |
15.0 (14.5–16.0)20.1 (18.2–22.9)
22.8 (20.4–25.2) 24.7 (21.8–29.6) 26.9 (22.8–31.8) 21.5 (19.0–24.2) 34.5 (32.1–43.3) |
14.5–16.019.0–23.0
25.0–30.0 38.0–41.0 |
Recommended sizes are based on the brochure provided by the manufacturer (Medicks Corporation, Tokushima, Japan).
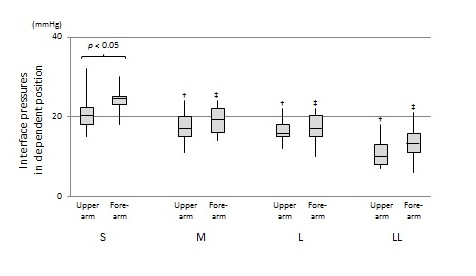
Figure 2.Interface pressures obtained by different compression sleeves.
†:p< 0.05 vs. IP on the upper arm with an S-sized sleeve on,‡:p< 0.05 vs. IP on the forearm with an S-sized sleeve on.
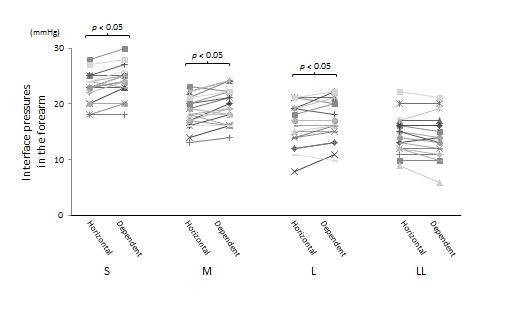
Figure 3.The change in interface pressures based on arm position.
In addition to increasingthe interstitial pressure by compression, acceleration of venous blood flow due to reduction of venous diameter reduces capillary filtration [11-13].However, it was reported that equivalent or even greater edema reduction was achieved with IP of 20-30 mmHg compared to IP of 44-68 mmHg in arm LE, possibly because of the impaired lymph drainage due to high IP in the latter [2,5]. It is also known that IP of > 10 mmHg may work to prevent and reduce edema, even in the leg [14]. These findings suggest that a lower IP may work to reduce edemaof the arm.If this is true, 2 or 3 size too large compression arm sleeves could work as shown in this study, and actually they work as we can experience in the clinic.
Although still controversial, a functional muscle pump may exist in the forearm, and may be enhanced by compression sleeves [15]. However, it is also known that the impact of gravity on venous pooling is insignificant in the arm, which was also supported by the current results [17].Moreover, structures like the solealveindo not exist in the forearm, and therefore, it is speculated that the impact of a compression sleeve to enhance a muscle pump may be limited.The idea of graduated compression is based on venous hemodynamics in the leg in static conditions, but the impact of graduated compression in the arm, particularly in dynamic states, has not been investigated thoroughly [18]. It was already reported that graduated compression was not always accomplished by ready-made arm sleeves [19]. Additionally, it has been suggested that a reverse-gradient stocking rather than a graduated,degressive compression stocking may be beneficial to improve venous hemodynamics in the leg [2]. Accordingly, the design of compression sleevesmay need to be reconsidered.Furthermore, it has been known that the increase in arm volume in LE is not only caused by fluid accumulation but also, and largely, by fat accumulation [20,21]. However, the impact of compression therapy on local fat metabolism has not yet been clarified.
Limitations
In this study, only IPs immediately after application of sleeves in healthy volunteers (presenting with different arm dimensions) were measured in a limited number of participants. Also, IP alone may not be a surrogate parameter both for the efficacy and tolerability of a compression sleeve. Therefore, it is difficult to draw definitive conclusions based on the study findings,and the conclusions might be oversimplified. The design of the currently employed arm sleeves is based on arm shapes in healthy Japanese adults including both males and females. However, we included only female participants in the current study, because patients with arm LE are predominantly female. This might result in the sleeves not fitting, particularly in the upper arm. However, considering the above situation, readjustment of the sleeve design by the manufacturer may be required.
Conclusion
In conclusion, even an arm sleeve that is 1 size too largecould lose IP of the therapeutic rangewhich is assumed to be> 20 mmHg, and designated graduation of IP. Unlike below-knee compression stockings, an appropriately sized compression sleeve may need to be prescribed for patients with arm LE.
Acknowledgements
None
Disclosure Statement
There are no conflicts of interest to declare.
Author Contributions
Study conception: Kotaro Suehiro<; Data collection: Kotaro Suehiro, Koshiro Ueda, Takashi Nagase, Makoto Samura, Yuriko Takeuchi andTakahiro Mizoguchi.
References
International Society of L. The diagnosis and treatment of peripheral lymphedema: 2013 Consensus Document of the International Society of Lymphology. Lymphol. 2013; 46: 1-11.
Flour M, Clark M, Partsch H, et al. Dogmas and controversies in compression therapy: report of an International Compression Club (ICC) meeting, Brussels, May 2011. Int Wound J. 2013; 10: 516-26.
Moseley AL, Carati CJ, Piller NB. A systematic review of common conservative therapies for arm lymphoedema secondary to breast cancer treatment. Ann Oncol. 2007; 18: 639-46.
Harris SR, Hugi MR, Olivotto IA, et al. Clinical practice guidelines for the care and treatment of breast cancer: 11. Lymphedema. CMAJ. 2001; 164: 191-9.
Damstra RJ, Partsch H. Compression therapy in breast cancer-related lymphedema: A randomized, controlled comparative study of relation between volume and interface pressure changes. J Vasc Surg. 2009; 49: 1256-63.
Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation. 2014; 130: 333-46.
Suehiro K, Morikage N, Murakami M, et al. Interface pressures derived from oversize compression stockings. Ann Vasc Dis. 2012; 5: 342-6.
Suehiro K, Morikage N, Yamashita O, et al. Adherence to and efficacy of different compression methods for treating chronic venous insufficiency in the elderly. Phlebology. 2016; 31: 723-8.
Földi M, Földi E. Földi’s Textbook of Lymphology: ELSEVIER; 2011; 504-5.
Jensen MR, Simonsen L, Karlsmark T, et al. Higher vascular endothelial growth factor-C concentration in plasma is associated with increased forearm capillary filtration capacity in breast cancer-related lymphedema. Physiol Rep. 2015; 3.
1Abu-Own A, Shami SK, Chittenden SJ, et al. Microangiopathy of the skin and the effect of leg compression in patients with chronic venous insufficiency. J Vasc Surg. 1994; 19: 1074-83.
Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res. 2010; 87: 198-210.
Belcaro G, Laurora G, Cesarone MR, et al. Microcirculatory effects of elastic stockings in diabetic microangiopathy: a 24-week study. J Cardiovasc Surg (Torino). 1993; 34: 479-82.
Stout N, Partsch H, Szolnoky G, et al. Chronic edema of the lower extremities: international consensus recommendations for compression therapy clinical research trials. Int Angiol. 2012; 31: 316-29.
Tschakovsky ME, Shoemaker JK, Hughson RL. Vasodilation and muscle pump contribution to immediate exercise hyperemia. Am J Physiol. 1996; 271: H1697-701.
Hirai M, Niimi K, Iwata H, et al. Comparison of stiffness and interface pressure during rest and exercise among various arm sleeves. Phlebology. 2010; 25: 196-200.
Meissner MH, Moneta G, Burnand K, et al. The hemodynamics and diagnosis of venous disease. J Vasc Surg. 2007; 46 Suppl S: 4S-24S.
Sigel B, Edelstein AL, Savitch L, et al. Type of compression for reducing venous stasis. A study of lower extremities during inactive recumbency. Arch Surg. 1975; 110: 171-5.
Williams AF, Williams AE. ‘Putting the pressure on’: a study of compression sleeves used in breast cancer-related lymphoedema. J Tissue Viability. 1999; 9: 89-94.
Brorson H, Ohlin K, Olsson G, et al. Adipose tissue dominates chronic arm lymphedema following breast cancer: an analysis using volume rendered CT images. Lymphatic research and biology. 2006; 4: 199-210.
Mortimer PS, Rockson SG. New developments in clinical aspects of lymphatic disease. J Clin Invest. 2014; 124: 915-21.
Received: February 02, 2018;
Accepted: February 10, 2018;
Published: February 13, 2018.
To cite this article : Kotaro Suehiro, Noriyasu Morikage, Koshiro Ueda, et al. Interface Pressures Derived from Oversized Compression Arm Sleeves. Japan Journal of Medicine. 2018; 1:1.
© Kotaro Suehiro, et al. 2018
