Mini Review / Open Access
DOI: 10.31488/jjm.1000140
Outcomes of Video-Assisted Thoracoscopic Surgery for Lung Cancer Performed by a Team of General Surgeons - A Retrospective Study
RyutaFukai*1, Tomoki Nishida1, Nao Kume2, Takaaki Murata2, Katsunori Miyake2, Naoko Isogai2, Rai Shimoyama2, Jun Kawachi2, Shyuji Kurata3, Hidekazu Iijima4, Kazunao Watanabe4
Department of General Thoarcic Surgery, Shonan Kamakura General Hospital, 1370-1 Okamoto, Kamakura, Kanagawa, 247-8533 Japan
Department of General Surgery, Shonan Kamakura General Hospital, 1370-1, Okamoto, Kamakura, Kanagawa, 247-8533 Japan
Department of General Surgery, Shonan Fujisawa Tokushukai Hospital, 1-5-1, Tsujidokandai Fujisawa, Kanagawa, 251-0041, Japan
Department of General Surgery, Tokyo Nishi Tokushukai Hospital, 3-1-1 Matsubara-choAkishima, Tokyo, 196-0003 Japan
*Corresponding author:RyutaFukai, Department of General Thoracic Surgery, Shonan Kamakura General Hospital, 1370-1, Okamoto, Kamakura, Kanagawa, 247-8533, Japan, Tel: +81 467 46 1717; Fax: +81 467 47 8243;
Abstract
Video-assisted thoracoscopic surgery (VATS) with hilar/mediastinal lymph node dissection for primary lung cancer is usually performed by teams of multiple thoracic surgeons. Few reports have documented the procedure being performed by a team of general surgeons. We introduced VATS lobectomy at our hospital for Stage I non-small cell lung cancer from August 2014, and this procedurewas performed by a team of general surgeons that also includedone thoracic surgeon. Our VATS lobectomy procedure isperformed via three ports;a camera port and two small instrumentation ports. We conducted fifty-six VATS lobectomies withhilar/mediastinal lymph node dissection (ND2a-1) for clinical stage I lung cancer. This report documents our perioperative and mid-term outcomes after these procedures to date. The perioperative results showed that operating timeswere virtually identicaland the rate of conversion to thoracotomy was lower than rates found in other studies, although blood loss was slightly higher. Mid-term disease-free survival and overall survival were tolerable. We believe that this procedure may become the standard form of surgical treatment for lung cancer at our hospital.
Keywords: video-assisted thoracoscopic surgery (VATS), general surgical team, lung cancer, perioperative outcome, disease-free survival, overall survival
Introduction
Reports have indicated that the long-term outcomes of clinical stage I lung cancer remain unchanged when surgery is performed via thoracotomy or thoracoscopy[1,2]. However, some studies have reported that long-term mortality was lower inpatients who underwent thoracoscopic surgery [3-6]. Moreover, close to 80% of all lung cancer patients in Japan who are eligible for surgery are in stage I [7], so thoracoscopic surgery is being performed more often to treat lung cancer. Video-assisted thoracoscopic surgery (VATS) with hilar/mediastinal lymph node dissection for primary lung cancer has become more popular over the last few decades, and it has been reported that patients treated with this surgical procedure experience significantly lower volumes ofblood loss[8]. VATS lobectomy is also reported to be associated with a lower incidence and severity of postoperative complications than standard thoracotomy in the elderly[8,9]. VATS lobectomy for primary lung cancer is increasingly being performed in Japan as its population continues to age.
However, thoracoscopic surgery to treat lung cancer is commonly performed by teams of thoracic surgeons, and reports indicate that a certain number of patients are required to standardize a technique [8, 10]. Moreover, there are very few reports of VATS lobectomy beingperformed by a team of general surgeons.
From August 2014, we introduced video-assisted thoracoscopic lobectomy, which was performed by a team of general surgeons that included one thoracic surgeon, and this paper documents a retrospective study comparing the perioperative outcomes after the introduction of thoracoscopic lobectomy at our hospital to those reported in the literature [2, 3].
Materials and Methods
Our subjects were 56 patients with clinical stage I lung cancer who underwent thoracoscopic lobectomy and hilar/mediastinal lymph node dissection (ND2a-1). These patients were selected from among 90 patients who underwent treatment for primary pulmonary malignant tumors from August 2014 until March 2016.
The primary endpoint was the duration of surgery, and the secondary endpoints were volume of blood loss, duration of the hospital stay, duration of postoperative drainage, and postoperative complications. The outcomes after the introduction of video-assisted thoracoscopic lung cancer surgery at our hospital were compared to those reported in the literature, and evaluated.
We also calculated the disease-free survival (DFS) and overall survival (OS) to evaluate oncological aspects of our operations. DFS was defined as the interval between the date of surgery and the date of relapse, and OS was defined as the interval between the date of surgery and the date of death.This study was approved by the institutional review board at our hospital.
Surgical method
A camera port was placed in the seventh intercostal space along the posterior axillary line, an accessory surgical port 2 – 3 cm in size was created at the inferior angle of the scapular (sixth or seventh intercostal space), a surgical port 3 – 5 cm in size was created from the middle to the anterior axillary line (fourth or fifth intercostal space), and the surgical port was covered with a wound protector (Figure 1).
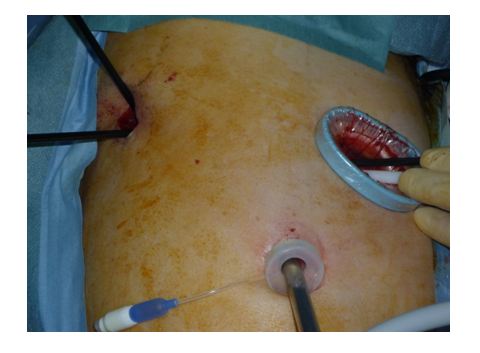
Figure 1.Surgical field during thoracoscopic right lower lobectomy: the camera port is at the bottom of the image, the accessory surgical port is in the left upper area and the surgical port is in the right upper area.
Vessels were managed by ligation after transection; ligature was performed using a knot pusher or an automated suture device, while bronchial and interlobular transection was performed using an automated suture device. The lymph node dissection was performed with a coagulator using thoracoscopy forceps or an energy device, and a Hem-o-lock(R) was used to manage a somewhat thick lymphatic duct.When a leak test was performed at the end of surgery that revealed a pulmonary fistula that required closure, we performed suture closure and reinforcement with polyglycolic acid felt and fibrin glue.
Statistical analysis
We calculatedthe DFS and OS in our surgical cases using the Kaplan-Meier method, and analyzed the data using EZR statistical software (64-bit version) [11].
Results
There were 28 men and 28 women included in the study with a mean age of 68.7 ± 8.4 years (41 – 82 years). The surgical technique was a right upper lobectomy in 20 cases, a middle lobectomy in four cases, a left upper lobectomy in ten cases, and a left lower lobectomy in ten cases.
The duration of surgery was 213 ± 69 minutes (100 – 443 minutes), the volume of blood loss was 354 ± 607 g (10 – 3400 g), and surgery on two cases was converted to thoracotomy intraoperatively. One subject underwent a right upper sleeve lobectomy to achieve complete resection of the cancer, and another subject had to undergo thoracotomy to ensure hemostasis of a pulmonary artery hemorrhage.
The mean duration of the postoperative hospital stay was 8.5 ± 3.8 days (5 – 25 days), and the mean duration of postoperative drainage was 4 ± 2.4 days (2 – 18 days). The tissue type was adenocarcinoma in 50 cases, squamous cell carcinoma in five cases, and adenosquamous carcinoma in one case. The postoperative complications were atrial fibrillation in three cases, wound infection in two cases, hoarseness in two cases, arterial thrombosis in two cases, a pulmonary fistula (≥ seven days) in one case, and an empyema in one case. There were no surgery-related deaths. The results are shown by comparison to the data in the literature (Table 1).
Table 1. Perioperative outcomes (data from when video-assisted lobectomy was introduced shown in comparison to the relevant literature)
| Cases | Age (years) | Operative duration(min.) | Blood loss(g) | Dissected lymph nodes (number) | Conversion to open thoracotomy (%) | Postoperative drainage(days) | Operative mortality | |
|---|---|---|---|---|---|---|---|---|
| Shigemura et al. | 50 | 66 | 246 | 96 | 23 | 12 | - | 0 |
| Whitson et al. | 59 | 67.1 | 228 | 251 | 6.3 | 15.7 | 5 | - |
| Our institution | 56 | 68.7 | 217 | 354 | 17 | 3.5 | 4 | 0 |
The mean observation period of all patients after curative operation was 40 months. The 4-year DFS of the patients whose postoperative course was followed up on an outpatient basis (n=55) was 72.1% (Figure 2).In addition, the 4-year survival of the patients in whom survival could be verified (n=52) was 98.1%, as shown in Fig. 3 (where ‘zero time’ was the date of surgery and December 31, 2018 was the end date).
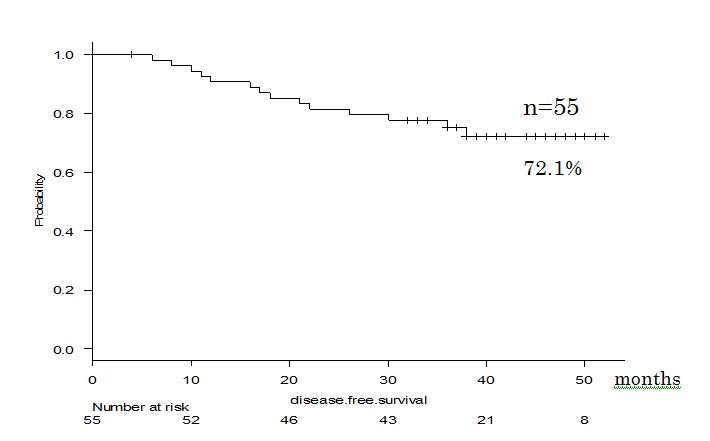
Figure 2.DFS curve of our cases.
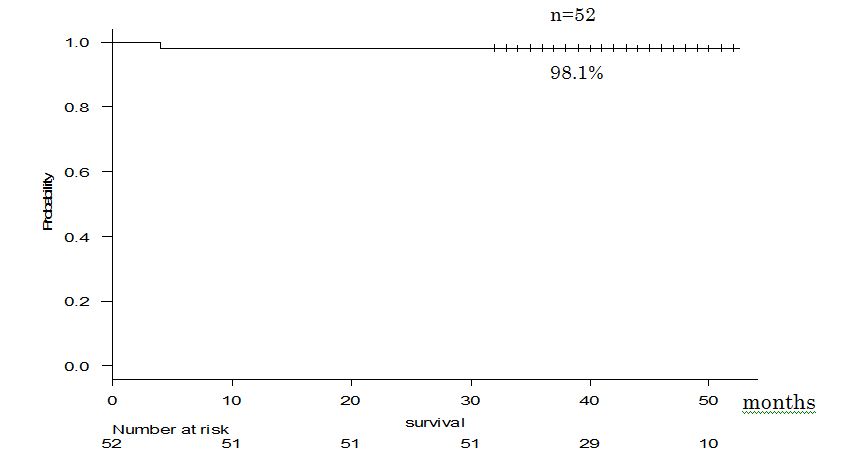
Figure 3.Survival curve of our cases.
Discussion
Thoracoscopic surgery to treat lung cancer is usually performed by a surgical team made up of multiple thoracic surgeons, and reports indicate that it takes roughly 50 patients to master the technique [8]. Most VATS lobectomy surgeries with hilar/mediastinal lymph node dissection are performed by a team of numerous thoracic surgeons. In the present study, we evaluated the perioperative outcomes of 56 subjects who underwent early video-assisted thoracoscopic surgery (VATS) and were clinical stage I patients treated by a team of general surgeons, which included one thoracic surgeon. The outcomes were then compared to literature reporting the introduction of VATS. There were no differences evident between our cases and those reported in the literature in terms of the duration of surgery and rate of conversion to thoracotomy. The mid-term oncological results (DFS, OS) were also comparable [2, 3]. We believe that this technique may be acceptable as a standard form of surgery to treat lung cancer at our hospital.
Surgery is performed through two instrumentation ports at our hospital; one for the surgeon (surgical port) and the other for the assistant (accessory surgical port). In addition to the camera port,the entire thoracoscopic lobectomy is therefore performed through a total of three ports, and is known as a Himeji-style VATS [1]. In this operation, similar to thoracotomy, countertraction generated by the surgeon and assistant in the surgical field are used to perform tissue dissection, and it is possible to perform dissection in the gaps between blood vessels using blunt dissection with gauze balls or a suction nozzle in tissues with no adhesions. In cases with narrow blood vessels or where it is difficult to introduce an automated suture device, ligation and transection is performed using a knot pusher. Since all doctors who perform VATS are able to confirm the status of the ligature, and are able to adjust the ligature position with the help of the assistant, we believe that this technique will be extremely useful once it has been mastered.
When we compared cases at our hospital to those reported in the literature, ours tended to have a somewhat higher volume of blood loss, so we reinvestigated the cases that required transfusion (Table 2).
Table 2. Patients who required transfusion
| Case | Cause of blood loss |
|---|---|
| 1 | The superior pulmonary vein was difficult to identify due to adhesions, and distal hemorrhage occurred after peripheral ligature and transection |
| 2 | The abnormal V4 – V5 bifurcations (following a course dorsal to the right bronchus) were injured during dissection of the bronchus |
| 3 | The patient developed a lung abscess after preoperative bronchoscopy, as well as marked adhesions to the chest wall |
| 4 | Hemorrhage occurred from calcified lymph nodes that were dissected after removal of the upper lobe, so the patient was converted to thoracotomy |
In case no. 1, the position of the left pulmonary vein was unknown because of adhesions, and the branches were exposed one after the other, then ligated and transected. Next, when the left upper lobe was retracted to enlarge the surgical field, the pulmonary vein was accidentally grasped, the distal ligature suture became unfastened and bleeding occurred. Inadequate attention was paid to the site that was grasped when the lung was retracted. In case no. 2, the branches V4-5, which bifurcated off the inferior pulmonary vein, were damaged during the dissection below the bronchial bifurcation, and bleeding occurred. Therefore, the understanding of the preoperative computed tomography (CT) scan was inadequately confirmed in the surgical field. In case no. 3, the patient developed a lung abscess after bronchoscopy, and required transfusion due to anemia, which was observed preoperatively, and intraoperative adhesion dissection. In case no. 4, after resection of the right upper lobe, hemorrhage occurred when the surgeon attempted to remove an inflammatory calcified lymph node attached to the A4 root. Reports indicate that dissection of hilar and perihilar vessels is contraindicated when calcification is present [10], so safety should be valued more than complete lymph node dissection in these types of cases. Excluding the cases that received transfusions, the mean volume of blood loss in the remaining 46 subjects was 211 g, which was consistent with the literature.
There were two cases (3.5%) in this study who were converted to thoracotomy, and the results were favorable when compared to reports in which thoracoscopic lobectomy was introduced (Shigemura et al. 12% [2], Whitson et al. 15.7% [3]). Among the subjects converted to thoracotomy, one subject exhibited lymphatic invasion of the right upper lobe root by a right upper lobe pulmonary adenocarcinoma. The surgery in this subject was converted to a right upper sleeve lobectomy. The other was the above-mentioned subject who suffered hemorrhage during the dissection of calcified lymph nodes (transfusion case no. 4). There were also other subjects who suffered hemorrhage during dissection of the pulmonary artery, although hemostasis was achieved by compressing the surface of the pulmonary parenchyma (for three minutes), and thereafter, it was possible to move to other sites in the surgical field and complete surgery without conversion to thoracotomy within 30 minutes. Meanwhile, in one case, hemorrhage occurred after the resected lung was removed and it was difficult to achieve adequate compression of the pulmonary artery hemorrhage site, so the subject was immediately converted to thoracotomy, and hemostasis was achieved (transfusion case no. 4). Despite our paucity of experience, it was difficult to control hemorrhage after the resected lung was removed, and when performing thoracoscopic surgery, we believe that adequate caution is required when there is hemorrhage during lymph node dissection after the resected lung has been removed.
There were several limitationsduring our study. Firstly, this was a small-scale, retrospective study performed immediately after the introduction of VATS lobectomy. Larger scale and prospective investigation are needed to facilitate more accurate evaluation. Second, our observation period was insufficient, and further follow-up is indispensable for assessing the true oncological value of our operations.
Our patients also developed a number of postoperative complications. These included two cases of irreversible hoarseness. In one case, the clip applied to the lymphatic duct damaged affected the recurrent laryngeal nerve when the subject underwent undergoing right upper lobectomy, and in the other case, a review of the surgical video for the subject undergoing a left upper lobectomy showed that tissue close to the recurrent laryngeal nerve had been coagulated, which may have been associated with the hoarseness. We need to be more attentive when performing lymph node dissection in order to avoid postoperative dysfunction, because the details of the thoracic anatomy are difficult to communicate among the team.
Conclusion
We performed a retrospective study of the perioperative outcomes after once we introduced video-assisted thoracoscopic lobectomy and hilar/mediastinal lymph node dissection (ND2a-1) at our hospital. Unlike several cases in the literature, surgery at our hospital was performed by a team of general surgeons with one thoracic surgeon. The duration of surgery was comparable to other studies, the rate of conversion to thoracotomy was low, and we believe that this technique may be acceptable as a standard form of surgery to treat lung cancer at our hospital. Meanwhile, the volume of blood loss was somewhat greater, and we observed complications associated with persistent postoperative dysfunction, i.e. hoarseness, so it would be desirable to increase the quality of our surgical techniques when dealing with hemorrhage and during lymph node dissection.
Acknowledgements
We would like to express our deepest gratitude to Takako Yamanashi, the Department of Surgery secretary at Shonan Kamakura General Hospital, for her unwavering cooperation during the collection of data for this report.Moreover, we thank Keiko Asou and MakotoUtsugi for their data acquisition and statistical analysis.
Conflicts of Interest
None
Abbreviations
VATS: Video-assisted thoracoscopic surgery; ND: Nodal dissection; DFS: Disease-free survival; OS: Overall survival
References
Yamamoto K, Ohsumi A, Kojima F, et al. Long-term survival after video-assisted thoracic surgery lobectomy for primary lung cancer. Ann Thorac Surg. 2010;89:353-359.
Shigemura N, Akashi A, Funaki S, et al. Long-term outcomes after a variety of video-assisted thoracoscopic lobectomy approaches for clinical stage IA lung cancer: a multi-institutional study. 2006;132:507-512
Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a sustematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg. 2008;86:2008-18.
Cheng D, Downey RJ, Kernstine K, et al. Video assisted thoracic surgery in lung cancer resection. Innovations. 2007;2:261-92.
Taioli E, Lee DS, Lesser M, et al. Long-term survival in video-assisted thoracoscopic lobectomy vs open lobectomy in lung-cancer patients: a meta-analysis. Eur J Cardiothorac Surg. 2013;44:591-97.
Li Z, Li H, Li L. Video-assisted thoracoscopic surgery versus open lobectomy for Stage I lung cancer: a meta-analysis of long term outcomes. ExpTher Med. 2012;3:886-892
Sawabata N, Miyaoka E, Asamura H, et al: Japanese lung cancer registry study of 11,663 surgical cases in 2004: demographic and prognosis changes over decade. J ThoracOncol. 2011;6:1229-1235
McKenna RJ. Complications and learing curves for video-assisted thoracic surgery lobectomy. ThoracSurgClin. 2008;18:275-80.
Port JL, Mirza FM, Lee PC, et al. Lobectomy in octogenarians with non-small cell lung cancer: Ramifications of increasing life expectancy and the benefits of minimally invasive surgery. Ann Thorac Surg. 2011;92:1951-7.
Gonfiotti A, Bongiolatti S, Borgianni S, et al. Development of a video-assisted thoracoscopic lobectomy program in a single institution: results before and after completion of the learning curve. 2016;3:450-453
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452-8.
Received: February 22, 2019;
Accepted: April 15, 2019;
Published: April 18, 2019.
To cite this article : Fukai R, Nishida T, NaoKume, et al. Outcomes of Video-Assisted Thoracoscopic Surgery for Lung Cancer Performed by a Team of General Surgeons – A Retrospective Study.Japan Journal of Medicine. 2019: 2:2.
©Fukai R, et al. 2019.
