Research Article / Open Access
DOI:10.31488/JJM. 167
Tesla Oscillator – Investigations on Its Beneficial Cell Effects
Peter C. Dartsch
Dartsch Scientific GmbH, Institut für zellbiologische Testsysteme, Auf der Vosshardt 25, D-49419 Wagenfeld, Germany
*Corresponding author: Prof. Dr. Peter C. Dartsch, Dartsch Scientific GmbH, Institut für zellbiologische Testsysteme, Auf der Vosshardt 25, D-49419 Wagenfeld, Germany.
Abstract
Background. High-frequency treatment with Tesla currents is a medical science that is ancient and has been forgotten over time. The Tesla Oscillator is based on the basic theory of Nikola Tesla and Georges Lakhovsky, but is a real technical advancement. It is stated by the manufacturer to be able to inform, structure or energize water which activates the body's own energy fields and stimulates the self-healing power. Materials and Methods. In the present study, current cell biological test meth-ods were used to investigate whether the Tesla experiment set, which includes the Tesla Oscillator and the handheld probe, is able to compensate an excess of endogenously generated radicals and to stimulate cell re-generation. Human promyelocytes (HL-60) were differentiated to functional neutrophils and were exposed to the frequencies of the Tesla Oscillator for a single time period up to 30 min. Then, superoxide anion generation was measured in the course of an induced oxidative burst. Connective tissue fibroblasts (L-929) were used to examine cell regeneration by closing a cell-free space due to migration and proliferation after exposure to the frequencies of the Tesla Oscillator for a single time period up to 30 min. Results. The results clearly demonstrate that the effects of a reduced radical generation after the exposure to the Tesla Oscillator was strictly time-dependent with a maximum of a nearly 40 % reduction after an exposure time of 30 min. The decrease in radi-cal generation was statistically significant for exposure times ≥ 15 min. In addition, also the morphological ex-amination of cell regeneration showed a clear time-dependent improvement in the closure of the cell-free space after a single exposure of the cells to the frequencies of the Tesla Oscillator. The maximum stimulation of cell regeneration in comparison to the untreated control was measured after the 30 min exposure time and was near-ly 30 % for exposure of the cells and 25 % for exposure of the culture medium. The stimulation was significant for exposure times ≥ 15 min. No significant difference was found between direct exposure of the cells or expo-sure of the culture medium which was used in the experiments afterwards. Conclusions. The use of the Tesla experiment set might improve and maintain personal health, vitality and well-being by an activation of cell regeneration and a reduced generation of superoxide anion radicals in complex wound healing or inflammatory processes.
Keywords: Tesla oscillator, tesla experi-ment set, oxidative burst, inflammation, functional neutrophil, connective tissue fibroblast, HL-60; L-929, cell regeneration, cell culture
Introduction
High-frequency treatment is a medical science that is ancient and has been forgotten over time [1,2]. The so-called Tesla currents were created in 1880 and were then implemented in high-frequency devices by various manufacturers. This therapeutic approach was further developed by Jacques-Arsène d’Arsonval in 1892 and used as a full-body treatment with high-frequency Tesla currents [3]. A further development was achieved by Kowarschick in Vienna [4].
The Tesla Oscillator is based on the basic theory of Nikola Tesla and Georges Lakhovsky, but it is no replica of the multi-wave oscillator according to Lakhovsky [2], but a real technical advancement. Its manufacturer, Wassermatrix AG, CH-6343 Rotkreuz, Switzerland, states that the Tesla Oscillator is able to inform, structure or energize water by using the handheld probe. The water energized in this way is then supplied to the body by drinking. This activates the body's own energy fields and stimulates the self-healing power. In this context, it must be taken into account that the human being consists of at least 70 % of water, so that a direct energizing of the body water is also possible. According to the manufacturer, many users of the device report amazing improvements in vitality, health and well-being.
In the present study, current cell biological test methods were used to investigate whether the Tesla experiment set, which includes the Tesla Oscillator and the handheld probe, is able to compensate an excess of endogenously generated radicals and thus prevent oxidative stress with a delayed healing process. For example, a local oxidative stress by reactive oxygen species in the tissue plays an important role in inflammatory processes [5-8] or cell regenerative processes in the course of wound healing [9,10] or after physical exercise or even overload [11-13]. It was also investigated whether the Tesla Oscillator has a direct beneficial influence on cell regeneration, either by energizing the cells themselves or by energizing the aqueous culture medium and causing a stimulation of cell migration and proliferation.
Material and Methods
Tesla Oscillator
A complete Tesla experiment set including the Tesla Oscillator and a handheld probe was kindly provided by Wassermatrix AG, CH-6343 Rotkreuz, Switzerland, for the duration of the experiments. The manufacturer states that the Tesla Oscillator is able to inform, structure or energize water by using the handheld probe. The water energized in this way is then supplied to the body by drinking. This activates the body's own energy fields and stimulates the self-healing power. In this context, it must be taken into account that the human being consists of at least 70 % of water, so that a direct energizing of the body water is also possible.
Cell culture
Human promyelocytes (cell line HL-60; ACC-3; ECACC 98070106; Leibniz-Institut; DSMZ German Collection for Microorganisms and Cell Cultures, Braunschweig, Germany) were cultivated over a period of several months. The cells were routinely cultivated in RPMI 1640 medium supplemented with 10 % growth supplement and 0.5 % gentamycin. The non-adherent cells were routinely cultivated as suspended mass cultures and were regularly subcultured twice a week with fresh culture medium. Cultures were incubated at 37 °C in an atmosphere of 5 % CO2 and 95 % air at almost 100 % humidity. By addition of 1.5 % dimethylsulfoxide to the culture medium, cells were differentiated over a period of 6 days into functional neutrophils, which are capable of generating superoxide anion radicals after addition of phorbol-12-myristate-13-acetate (Sigma-Aldrich, Deisenhofen, Germany) [14-18].
Connective tissue fibroblasts (cell line L-929; ACC-2; Leibniz Institute DSMZ - German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany) were routinely grown in RPMI 1640 with 10 % growth supplement and 0.5 % gentamycin and incubated in an incubator at 37 °C in a humidified atmosphere of 5 % CO2 and 95 % air. All cell culture reagents were purchased from Pan-Biotech, Aidenbach, Germany.
Experimental design for cell treatment

Figure 1:Experimental setup for the exposure of functional neutrophils to investigate the formation of endogenously generated superoxide anion radicals (left picture), of connective tissue fibroblasts to investigate cell regeneration (middle picture) and by energizing the culture medium used for cell regeneration of connective tissue fibroblasts (right picture).
The hand probe was clamped vertically outside the incubator with the green antenna rod pointing downwards in a table stand. Treated cell cultures or test tubes made of polypropylene with the cells or water to be informed were placed in a circle directly around the antenna rod (Figure 1). The corresponding controls were located for the duration of the exposure in another room at least 10 meters away. The exposure was conducted at room temperature for t = 0 (untreated control), 5, 15 and 30 min.
Radical generation by functional neutrophils
Cells were cultivated as suspended mass cultures in special culture flasks with a ventilated lid (25 cm2 growth area; TPP, Switzerland) so that an atmospheric gas exchange can be inhibited by turning the lid into another position. Thus, any pH changes in the bioassay during exposure to the frequencies of the Tesla Oscillator could be excluded.
On day 5 of differentiation, cells were exposed to the frequencies of the Tesla Oscillator by the hand probe as described above and incubated for another 16 h in the incubator. The flasks were carefully wrapped in several layers of aluminium foil to avoid any unwanted interactions between the exposed cells and the unexposed controls.
Finally, the cells of each experimental series were washed by several centrifugation steps (6 min at 190 x g) and repeated washings in phosphate buffered saline with calcium and magnesium. Cells were resuspended in phosphate buffered saline with calcium and magnesium containing 10 mM glucose. 60 µl of the cell suspension aliquots were pipetted to a reaction mixture containing the tetrazolium dye WST-1 (Sigma-Aldrich, Deisenhofen, Germany) and phorbol-12-myristate-13-acetate for the induction of an oxidative burst. The reactive superoxide anion radicals in the reaction mixture caused the cleavage and color change of the dye. The amount of superoxide anion radicals present in the reaction mixture was directly related to this color change. The optical density was recorded at various time points up to 45 min as a differential measurement ∆OD = 450 – 690 nm by an Elisareader (BioTek ELx 808 with software Gen 5 version 3.00) and calculated with Microsoft Excel 2016.
Regeneration of connective tissue fibroblasts
Cells were seeded at a density of 100,000 cells/ml into the four cell culture compartments of silicone Culture-Insert 4 Well (ibidi, Gräfelfing, Germany). The four compartments are separated by a 500 μm thick silicone wall. Due to their especially designed surface, the inserts stick to the surface firmly and completely prevent any cell attachment and growth under their silicone walls.
When the silicone frame is removed, a cell-free space (artificial wound) with sharp edges is left. The cell-free space can then be closed by cell migration and proliferation.
48 h after seeding, the cell layers have become confluent and the silicone frames were removed. Immediately after removing the silicone frame, cell cultures were exposed once for t = 0 (untreated control), 5, 15 and 30 min to the frequencies of the Tesla Oscillator and then incubated for 20 h. In an additional series of experiments, not the cell cultures themselves, but the aqueous culture medium was exposed to the frequencies of the Tesla experiment set for t = 0 (untreated control), 5, 15 and 30 min and then pipetted to the cells before another 20 h incubation was performed. All cell samples were shielded by multiple wrapping in aluminum foil and placed at different locations within the incubator to avoid any unwanted interactions between exposed and unexposed cells. Finally, cells were fixed with methanol and stained with Giemsa’s azur eosin methylene blue solution (Merck, Darmstadt, Germany) and air-dried. The width of the remaining cell-free space was measured at 12 different locations of each sample. Three independent experiments were conducted.
Statistical analysis
Statistical analysis was done by using the non-parametric, two-tailed Wilcoxon-Mann-Whitney test.
Results
As shown in Figure 2, the reduced generation of endogenous radicals after exposure to the Tesla experiment set was strictly time-dependent. Even after a short exposure time of only 5 min, the generation of free radicals was reduced by 11.2 ± 5.5% (mean value ± standard error of the mean) in comparison to the untreated control. After an exposure time of 30 min with the Tesla experiment set the reduced generation of radicals was 37.7 ± 7.7% (mean value ± standard error of the mean). The decrease in radical generation was statistically significant (p ≤ 0.01) for exposure times ≥ 15 min.
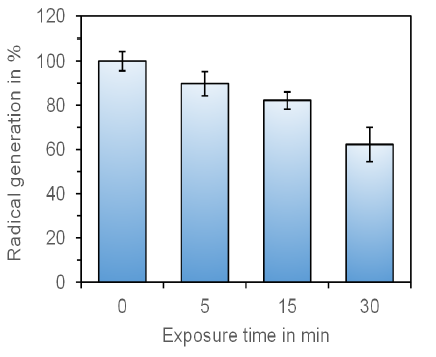
Figure 2:Representation of the time-dependent endogenous radical formation of cell cultures treated with the Tesla Oscillator. The decrease in radical generation is statistically significant for exposure times ≥ 15 min (p ≤ 0.01; Wilcoxon-Mann-Whitney test). The untreated control was set as “100%”. The data represent the mean value ± standard error of the mean of three independent experiments with duplicates.
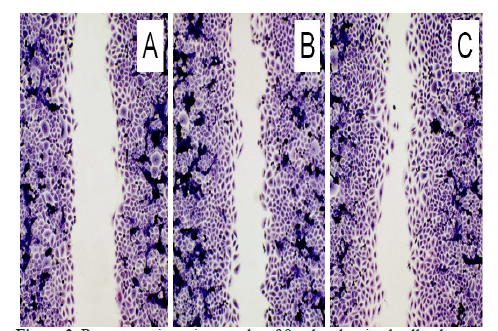
Figure 3:Representative micrographs of fixed and stained cell cultures demonstrating the closure of the cell-free space within 20 h. (A) Untreated control; (B) Cell culture which was directly exposed to the Tesla oscillator; (C) Cell culture with culture medium which was exposed to the Tesla Oscillator. Note the reduced cell-free space after both treatments. Olympus IX-50 inverted microscope equipped with an Olympus planachromate 10x and an Olympus E-10 digital camera with 4 megapixel resolution at bright field illumination.
Also the morphological examination of cell regeneration with connective tissue fibroblasts showed a clear improvement in the closure of the cell-free space after a single exposure of the cells to the frequencies of the Tesla experiment set (Figure 3). The evaluation of the cell-free space resulted in a more differentiated view on the time-dependent beneficial effect (Figure 4). There was no difference between the direct exposure of the cell cultures or by exposure of the culture medium which was then added for the cell regeneration test.
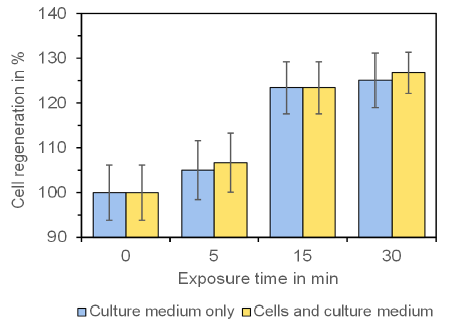
Figure 4:Effect of the Tesla Oscillator on the regeneration of connective tissue fi-broblasts. The stimulation of cell regeneration with increasing exposure time is clearly visible and is statistically significant for exposure times ≥ 15 min (p ≤ 0.01; Wilcoxon-Mann-Whitney test). The untreated control was set as “100%”. Note that there is no difference between a direct exposure of the cells or an exposure of the culture medium which was then added to the cells. Data represent mean value ± standard error of the mean of three independent experiments.
The maximum stimulation of cell regeneration in comparison to the untreated control was measured after the 30 min exposure time and was 26.8 ± 4.5 % (mean value ± standard error of the mean) for exposure of the cells and 25.1 ± 6.1 % (mean value ± standard error of the mean) for exposure of the culture medium. The stimulation was statistically significant for exposure times ≥ 15 min (p ≤ 0.01).
Discussion
Cell cultures are frequently used for a better understanding of the mechanisms that underlie cell activity in vivo. This includes differentiation, migration, proliferation, and
Metabolism [19,20]. For over a century, two-dimensional cell cultures as used in this present study, have been used as in vitro models and are well accepted in preclinical research. However, one might argue that cell cultures do not represent the human body with its complex metabolic pathways and facets, but it should be considered that cell cultures can shed light on selected aspects of living matter and are a useful tool to avoid animal experiments.
Neutrophils are the most abundant type of granulocytes which make up about 60 % of all white blood cells in humans and are normally found in the bloodstream. They form an essential part of the innate immune system [21] and, therefore, play a key role in the front-line defense against invading microbial pathogens. However, neutrophils are also one of the first responders of inflammation and migrate from the blood into the inflamed tissue and generate highly reactive superoxide anion radicals in the course of an oxidative or respiratory burst [5,22]. The present study with functional neutrophils has shown that the frequencies of the Tesla experiment set are able to reduce the generation of unwanted superoxide anion radicals by functional neutrophils in a time-dependent manner after only one single application of the Tesla Oscillator. Thus, a (chronic) inflammatory process in the tissue might be improved by the inhibition of radical generation in the inflamed tissue.
Somehow synergistically is the beneficial effect of the frequencies of the Tesla Oscillator on cell regeneration which is stimulated in a time-dependent manner. In this present test system, especially the granulation phase is simulated which is characterized by migration and proliferation of the cells in order to close the cell-free space (= wounded area) in vivo. However, a complicated wound healing process is also related to local oxidative stress [23-25] which is reduced by the frequencies at the same time.
The results clearly demonstrate that the frequencies of the Tesla Oscillator are able to inform, structure or energize the water of the aqeous culture medium surrounding the cells. Therefore, one might conclude that our body which consists of at least 70 % of water is also influenced by the frequencies, either by a direct influence on the cells or indirectly by the body fluid. As a matter of fact, the use of the Tesla experiment set might improve and maintain personal health and well-being by an activation of cell regeneration and a reduced generation of superoxide anion radicals in complex wound healing or inflammatory processes.
References
1. Paul E. Fortschritte der Hochfrequenz-Therapie nebst neuen Behandlungsvorschriften. Verlag Bad Aussee E. Paul. 100. Auflage. 1930.
2. Lakhovsky G. Der Multiwellen Oszillator. Copyright by Georges Lakhovsky, Paris. 1934.
3. Simonis WC. Die Hochfrequenztherapie von Arsonval bis Zeileis. Verlag der Aerztlichen Rundschau Otto Gmelin, München. 1930.
4. Kowarschik J. Die Elektrotherapie. In: Physikalische Therapie. Springer, Wien. 1957; 107-175.
5. Nathan C. Points of control in inflammation. Nature. 2002; 420:846–852.
6. Nathan C, Ding A. Nonresolving inflammation. Cell. 2010; 140:871-882.
7. Pawelec G, Goldeck D, Derhovanessian E. Inflammation, ageing and chronic disease. Curr Opinion Immunol. 2014; 29:23-28.
8. Kotas ME, Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell. 2015; 160:816-827.
9. Clark RAF. Wound repair: overview and general considerations. In: The Molecular and Cellular Biology of Wound Repair, Clark RAF (Ed.), 2nd edition, Plenum Press, New York, NY, 3-50; 1996.
10. Keller auf dem U, Kümin A, Braun S, et al. Reactive oxygen species and their detoxification in healing skin wounds. J Investigat Dermatol Symp Proc. 2006; 11:106-111.
11. Apor P, Rádi A. [Physical exercise, oxidative stress and damage]. Orvosi Hetilap. 2006; 147:1025-1031.
12. Vollaard NB, Cooper CE, Shearman JP. Exercise-induced oxidative stress in overload training and tapering. Med Sci Sports Exercise. 2006; 38:1335-1341.
13. Larsen MK, Matchkov VV. Hypertension and physical exercise: The role of oxidative stress. Medicina. 2016; 52:19-27.
14. Babior BM. NADPH oxidase: An update. Blood. 1999; 93:1464-1476.
15. Tan AS, Berridge MV. Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt WST-1 to produce a soluble formazan: a simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J Immunol Meth. 2000; 238:59-68.
16. Teufelhofer O, Weiss RM, Parzefall W, et al. Promyelocytic HL60 cells express NADPH oxidase and are excellent targets in a rapid spectrophotometric microplate assay for extracellular superoxide. Toxicol Sci. 2003; 76:376-383.
17. Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002; 82:47-95.
18. Dartsch PC. TIIOS – a sensitive and cell-based test assay for the screening of biologically active substances for their antioxidant potential. Innov Food Technol. 2006; 32:72-75.
19. Huh D, Hamilton GA, Ingber DE. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011; 21:745–754.
20. Duval K, Grover H, Han L-H, et al. Modeling physiological events in 2D vs. 3D cell culture. Physiol. 2017; 32:266-277.
21. Witko-Sarsat V, Rieu P, Descamps-Latscha B, et al. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest. 2000; 80:617-653.
22. Ward PA. The acute inflammatory response and its regulation. Arch Surg. 1999; 134:666-669.
23. Cano Sanchez M, Lancel S, Bou-langer E, et al. Targeting oxidative stress and mitochondrial dysfunction in the treatment of im-paired wound healing: A systematic review. Antioxidants. 2018; 7:98.
24. Schafer M, Werner S. Oxidative stress in normal and impaired wound repair. Pharmacol Res. 2008; 58:165–171.
25. Dunnill C, Patton T, Brennan J, et al. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int Wound J. 2017; 14:89-96.
Received: June 08, 2021.
Accepted: June 14, 2021.
Published: June 16, 2021 .
To cite this article : Peter C. Dartsch. Tesla Oscillator – Investigations on Its Beneficial Cell Effects. Japan Journal of Medicine. 2021; 4:1.
© 2021 Peter C. Dartsch.
